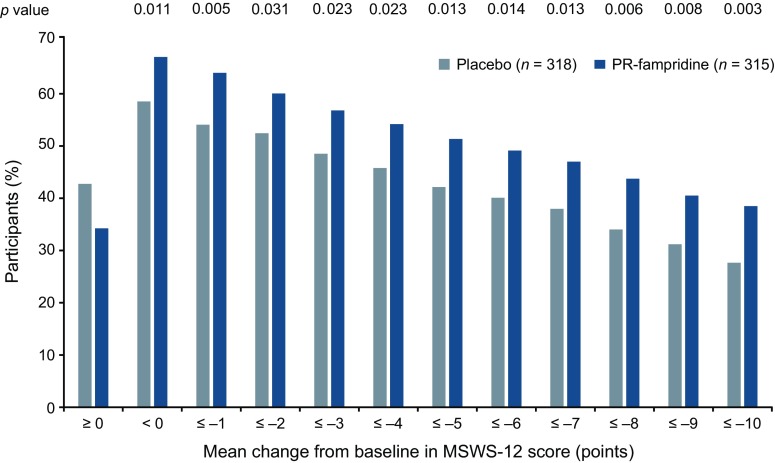
Fig. 2.
Estimated proportion of study participants who met each threshold of mean MSWS-12 score change over 24 weeks in the modified intention-to-treat sample. The MSWS-12 was transformed to a 0–100 scale; higher score = greater walking limitation. Negative change indicates improvement. Estimated percentages were based on binomial proportions. Multiple imputation was used for missing post-baseline data. Nominal p-values for PR-fampridine vs. placebo are from a logistic regression model adjusted for covariates (see Sect. 2). MSWS-12 12-item Multiple Sclerosis Walking Scale, PR prolonged-release