Figure 2.
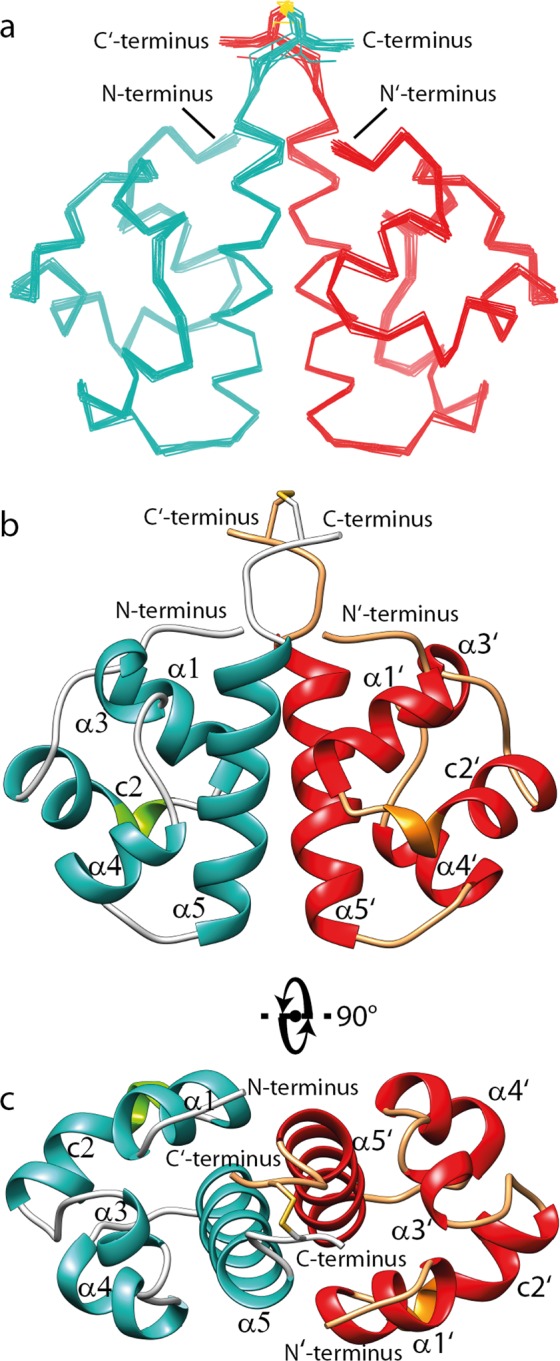
NMR solution structure of SLy1 SAMC homodimer. (a) Superposition of the 15 lowest energy structures of the disulphide bond-stabilized homodimer of SAMC. The backbones of the monomers are coloured teal and red. (b,c) Ribbon representation of the structure closest to the average backbone structure (r.m.s.d. = 0.19 Å) of the ensemble. α-helices are shown in teal and red in each monomer, whereas the 310-helix part in the composite helix c2 is shown in green and orange. Helices α5 and α5′ are in tight contact to form the major part of the interface. They run in a parallel fashion with an angle of ~−50° between their long axes. Helix α1 and the N-terminus of one monomer are in close proximity to the C-terminal region of helix α5′ of the opposing monomer, and also form part of the dimer interface.
