Abstract
Ribavirin, once a staple of hepatitis C treatment, has significant drawbacks, including treatment-limiting side effects, the requirement for intensive laboratory monitoring, the need for frequent dose adjustments, and teratogenicity. These factors make it difficult to escalate ribavirin-based HCV treatment to most infected patients globally. Most studies have shown comparable response rates between ribavirin-inclusive and ribavirin-sparing regimens in uncomplicated patient populations. However, ribavirin is still used in the management of patients who have failed previous therapy as well as those with decompensated liver disease. In this review, we explore the evidence supporting the use of ribavirin in the current climate of hepatitis C treatment with oral combination direct-acting antiviral agents.
Keywords: Chronic hepatitis C, Direct-acting antivirals, Ribavirin
Introduction
Hepatitis C virus (HCV) infection affects 71 million people worldwide1 and is a leading cause of death globally.2 Prior to the approval of oral combination direct-acting antivirals (DAAs) in 2014, all recommended HCV treatments included pegylated-interferon α (PEG-IFNα) and ribavirin (RBV). In a recent systematic review and meta-analysis on hepatitis C treatment rates, however, it was estimated that only 25% of patients with chronic HCV infection worldwide were treated with these immune-based therapies.3 Treatment rates were likely limited by a number of factors, including the significant rates of side effects, the lack of adequate numbers of specialist providers, the fact that these therapies were contraindicated in patients with decompensated liver disease, and limited efficacy, particularly in patients with HIV/HCV co-infection or those with advanced liver disease.
Now, interferon-sparing combination DAA regimens have dramatically improved the treatment of HCV, with higher treatment efficacy, improved tolerability, and ease of dosing, with the majority of regimens available in once daily oral dosing.4 With the first published trials showing comparable response rates to combination DAA-based HCV treatments when given with and without RBV, it has been suggested that RBV-sparing regimens would improve treatment safety, tolerability, and adherence.5–7 However, there are several clinical scenarios and treatment regimens in which the addition of RBV is still recommended. Here, we discuss the data behind the current guidelines regarding the use of RBV in the revolutionary age of DAA-based treatment for HCV.
RBV
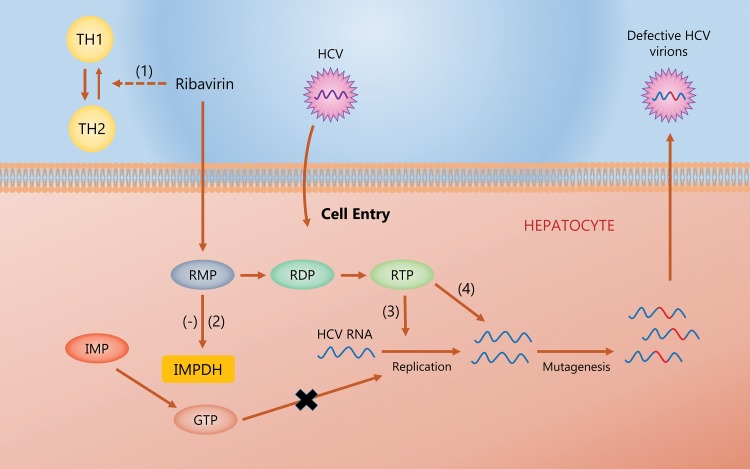
RBV is a synthetic triazole guanosine analog with activity against both DNA and RNA viruses.8,9 Suggested mechanisms of RBV include modulation of T helper-1 and -2 lymphocyte imbalance, depletion of cell guanosine triphosphate by inhibiting inosine monophosphate dehydrogenase, inhibiting the viral RNA-dependent RNA polymerase, impairment of translation by preventing the capping of messenger viral RNA, and lethal viral mutagenesis (Fig. 1).8,9 Before the introduction of RBV, PEG-IFNα was the only available treatment for HCV, achieving low rates of sustained virologic response (SVR)—defined as absence of plasma HCV RNA in blood at 12 or 24 weeks after stopping treatment—of 15–20%.8 The addition of RBV to PEG-IFNα was shown to be superior to the use of PEG-IFNα alone,8,10–13 with an increased SVR rate of approximately 30%8,10 among patients who received a regimen with RBV. The combination of PEG-IFNα and RBV also reduced the risk of post-treatment viral relapse.8 In a study by McHutchinson et al.,8 relapse rates were reduced by 38% in patients who received PEG-IFNα and RBV for 24 weeks and by 22% in those who received the regimen for 48 weeks.8 Due to higher efficacy and decreased rates of relapse compared to PEG-IFNα regimens alone, PEG-IFNα in combination with RBV became the standard of care for hepatitis C treatment in the 1990s and early 2000s.
Fig. 1. Proposed mechanisms of RBV.
(1) Immunomodulation favoring TH2 over the TH1 phenotype to enhance host immunity to the virus. (2) Competitive inhibition of IMPDH and subsequent depletion of the GTP pool, limiting replication of viral genomes and viral protein synthesis. (3) Inhibition of post-translational mRNA capping by directly inhibiting viral mRNA polymerase. (4) RBV acts as a mutagen in the target virus.
Abbreviations: IMPDH, inosine monophosphate dehydrogenase; RMP, ribavirin monophosphate; RDP, ribavirin diphosphate; RTP, ribavirin triphosphate; TH1, T helper 1; TH2, T helper 2.
Although RBV augments the effects of PEG-IFNα in HCV treatment, there are side effects associated with its use that significantly alter patient adherence and tolerance. Hemolytic anemia is the most significant adverse effect,12,14 afflicting up to 10% of patients,15 requiring laboratory monitoring of hemoglobin while on treatment, and frequent dose adjustments. Other side effects include pruritis, fatigue, and upper respiratory symptoms.14 Additionally, RBV is teratogenic, so women of child-bearing age who are on the medication or who have male partners taking the medication must use contraception.16,17
DAAs
In May 2011, the FDA approved the protease inhibitors boceprevir and telaprevir, which were found to increase the rates of SVR 12 weeks after end of treatment (SVR12) when combined with PEG-IFNα and RBV,18,19 and the standard of care for hepatitis C treatment shifted to include one of these oral medications in combination with PEG-IFNα and RBV. Though the protease inhibitors greatly improved HCV treatment, their use was restricted by complicated dosing strategies requiring response-guided therapy and lead-in treatments, a low genetic barrier to resistance, and extensive drug-drug interactions. In 2013, the second generation of DAAs, which included the nucleotide analogue sofosbuvir (SOF), came to the forefront of HCV treatment. When combined with PEG-IFNα and RBV, treatment with SOF produced SVR12 rates in excess of 90%.6,20–22 Daclatasvir (DCV), a non-nucleoside RNA polymerase inhibitor, in combination with PEG-IFNα and RBV also demonstrated high antiviral potency.23 In light of these near total response rates, investigators began to hypothesize that use of RBV could be eliminated altogether.
Several studies published in 2014 demonstrated that RBV-free treatment regimens, composed of only DAAs, were highly efficacious. The first, by Sulkowski et al.7 (AI444040 Study), treated 211 patients with either genotype (GT) 1, 2, or 3 infection with a combination of DCV and SOF with or without RBV for varying treatment durations, including 12 and 24 weeks. Among the GT1 treatment-naïve patients, a combination of DCV and SOF for 12 weeks produced SVR12 rates of 100% (41/41), compared to a 95% (39/41) SVR12 rate in those patients treated with DCV+SOF+RBV for 12 weeks. Among the GT1 patients who had failed previous treatment with a protease inhibitor, SVR12 rates were 100% (21/21) in patients treated with DCV+SOF for 24 weeks, and 95% (19/21) for patients treated with DCV+SOF+RBV for 24 weeks. Similarly, among patients with GT2 or GT3 infection, the addition of RBV did not result in a significantly higher virologic response rate; SVR12 was 100% (14/14) and 86% (12/14) for patients who were administered the RBV-sparing and RBV-inclusive regimens, respectively.7 The lower SVR rates seen with the RBV-inclusive regimens may reflect the high potency of DCV and SOF, indicating that RBV does not add to the potency of these oral DAAs.
A second study, LONESTAR,6 assessed treatment of GT1 infection in patients who were treatment-naive or previously treated with a protease-inhibitor regimen. There were 19 treatment-naïve patients treated with LDV/SOF for 12 weeks, and 18 (95%) achieved SVR12. Among 21 patients who had failed previous treatment with a protease inhibitor, all (100%) achieved SVR12 after 12 weeks of LDV/SOF+RBV and 18 of 19 (95%) patients treated with LDV/SOF alone achieved SVR12. In a phase 3 study by Afdhal et al.5 (ION-1), 865 treatment-naïve patients with GT1 infection were randomized to receive LDV/SOF for 12 weeks, LDV/SOF+RBV for 12 weeks, LDV/SOF for 24 weeks, or LDV/SOF+RBV for 24 weeks. The rates of SVR12 were 99% in the group that received 12 weeks of LDV/SOF, 97% in the group that received 12 weeks of LDV/SOF+RBV, 98% in the group that received 24 weeks of LDV/SOF, and 99% in the group that received 24 weeks of LDV/SOF+RBV.
Last, a phase 2b study24 evaluated the efficacy of a NS3/4A protease inhibitor (ABT-450) boosted with ritonavir (ABT-450/r), a non-nucleoside polymerase inhibitor (ABT-333), and a NS5A inhibitor (ABT-267) in various combinations with or without RBV. A total of 571 non-cirrhotic patients with GT1 infection (438 treatment-naive and 133 treatment-experienced) underwent randomization to receive a regimen of ABT-450/r, combined with ABT-267 and/or ABT-333, for 8, 12 or 24 weeks and received at least one dose of therapy. All the subgroups but one also received RBV (dose determined according to body weight). When comparing the SVR12 rate of the group that received all three drugs without RBV for 12 weeks and the group that received all three drugs with RBV for 12 weeks (89% vs. 96%, respectively), the contribution of RBV was not significant (p = 0.09). These studies suggest that adding RBV to DAAs does not result in significantly higher SVR12 rates, and are summarized in Table 1.
Table 1. HCV treatment studies and comparison of SVR rates of RBV-containing regimens.
| Study | Regimen | SVR Rate |
| Lawitz et al.50 | SOF+PEG-IFNα+RBV vs. Placebo+PEG-IFNα+RBV | 91% vs. 58% |
| Jacobson et al.51 | SOF+RBV for 12 weeks vs. placebo | 78% vs. 0% |
| ATOMIC52 | SOF+PEG-IFNα+RBV for 12 weeks vs. SOF+PEG-IFNα+RBV for 24 weeks | 90% vs. 93% |
| Osinusi et al.53 | SOF+weight-based RBV vs. SOF+low-dose RBV | 68% vs. 48% |
| Pol et al.23 | PEG-IFNα+RBV vs. DCV+PEG-IFNα+RBV | 25% vs. 83% |
| Sulkowski et al. (AI444040)7 | DCV+SOF for 12 weeks vs. DCV+SOF+RBV for 12 weeks | 100% vs. 95% |
| DCV+SOF for 24 weeks vs. DCV+SOF+RBV for 24 weeks | 100% vs. 95% | |
| LONESTAR6 | LDV+SOF for 12 weeks vs. LDV+SOF+RBV for 12 weeks | 95% vs. 100% |
| ION-15 | LDV+SOF for 12 weeks vs. LDV+SOF+RBV for 12 weeks | 99% vs. 97% |
| LDV+SOF for 12 weeks vs. LDV+SOF+RBV for 12 weeks | 98% vs. 99% | |
| Kowdley et al.24 | ABT-450/r+ABT-333+ABT-267 for 12 weeks vs. ABT-450/r+ABT-333+ABT-267+RBV for 12 weeks | 89% vs. 96% |
Studies directly comparing DAAs are lacking, but a recent meta-analysis25 comparing different DAA-based regimens, with and without RBV, showed no improvement in treatment efficacy among DAA regimens that included RBV over those that were RBV-sparing. In addition, treatment regimens that incorporated RBV had a higher relative risk of adverse events, including anemia and fatigue. These studies support limiting the use of RBV, given the lack of increase in efficacy and more frequent toxicity. However, as we explore below, there are scenarios in which the addition of RBV to a DAA-based regimen might be warranted.
Roles of RBV
There are several scenarios and patient groups in which the use of RBV is necessitated, as outlined below and summarized in Table 2.
Table 2. Indications/clinical scenarios and supporting studies which warrant use of ribavirin.
| Patient Population | Regimen | Study |
| GT1a and GT1b, non-cirrhotic, treatment-naïve | PrOD+RBV for 12 weeks | SAPPHIRE-I26 |
| GT1a, non-cirrhotic, treatment-naïve | PrOD+RBV for 12 weeks | PEARL-IV27 |
| GT1, non-cirrhotic, treatment-experienced | PrOD+RBV for 12 weeks | SAPPHIRE-228 |
| GT1a, with or without compensated cirrhosis, treatment-naïve or treatment-experienced with protease inhibitor, with baseline NS5A RASs | EBR/GZR+RBV for 16 weeks | C-EDGE29,30 and C-SALVAGE31 |
| GT1, compensated cirrhosis, treatment-experienced with interferon | LDV/SOF+RBV for 12 weeks | SIRIUS32 |
| GT3, compensated cirrhosis, treatment-experienced with interferon | SOF/VEL+RBV for 12 weeks | Pianko et al.33 |
| GT4, non-cirrhotic, treatment-naïve and treatment-experienced | PrO+RBV for 12 weeks | PEARL-I34 |
| All genotypes, decompensated cirrhosis, treatment-naïve and treatment-experienced (except with NS5A or NS5B inhibitor) | SOF/VEL+RBV for 12 weeks | ASTRAL-435 |
| Post-liver transplant GT 1, 4, 5, 6 (including decompensated cirrhosis) | LDV/SOF+RBV for 12 weeks | SOLAR-1,36 HCV-TARGET,37 and Kwok et al.38 |
| Post-liver transplant GT 2, 3 (compensated cirrhosis only) | DCV/SOF+RBV for 12 weeks | ALLY-1,39 and Fontana et al.40 |
Augmenting viral response in GT1 patients
RBV has been efficacious when used in regimens for both treatment-naïve and treatment-experienced patients with GT1 infection. The daily fixed-dose combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD), for example, is an alternative regimen approved for treatment-naïve GT1a and 1b patients with or without cirrhosis when given with weight-based RBV. The efficacy of RBV when added to PrOD for HCV treatment, especially in GT1a patients, was demonstrated in three trials. In the SAPPHIRE-I trial,26 631 treatment-naïve, non-cirrhotic patients with GT1 infection were treated with PrOD+weight-based RBV for 12 weeks. Rates of response were more than 95% among the treatment-naïve patients with GT1 infection. Additionally, in the PEARL-IV study, Ferenci et al.27 evaluated the efficacy of patients treated with PrOD with or without RBV. While there was no difference in rates of virologic failure with or without RBV among the GT1b patients, among the GT1a patients the rate of virologic failure was higher in the RBV-free group compared to the RBV group (7.8% vs. 2.0%). Last, for patients who had previously been unsuccessfully treated with PEG-IFNα and RBV, the SAPPHIRE-2 study28 examined the use of PrOD with weight-based RBV in non-cirrhotic GT1 patients. In that study, patients were treated for 12 weeks and had an overall SVR12 rate of 96%, with no differences noted when comparing response rates between subtypes.
RBV is also used to augment the SVR rate in patients who have baseline HCV resistance, when added to the fixed-dose combination of elbasvir (EBR) and grazoprevir (GZR). In the phase 3 C-EDGE trial,29 an international multicenter study, a 12- or 16-week course of EBR/GZR was given to treatment-naïve patients infected with HCV genotypes 1, 4 or 6, some of whom had compensated cirrhosis. Of the 421 patients, 382 (91%) had GT1 infection. The SVR12 was 92% in GT1a patients and 99% in GT1b patients. However, a post-hoc subanalysis revealed that the presence of baseline NS5A resistance-associated substitutions (RASs) was significantly associated with a decreased virologic response among GT1a patients; of 154 GT1a patients, 19 (12%) had baseline NS5A RASs, and of these 19, only 11 (58%) achieved SVR12 compared to 133/135 (99%) patients without NS5A RASs.
Kwo et al.30 extrapolated data from the C-EDGE trial and found that among 58 patients who received EBR/GZR for 16 weeks with RBV, there were no virologic failures. Based on these analyses which suggest that prolonging treatment and adding RBV can overcome reduced EBR susceptibility, NS5A resistance testing is recommended for any GT1a patient in whom EBR/GZR therapy is considered. Use of RBV for the presence of baseline RASs is also recommended for treatment-experienced patients with GT1 infection (with or without cirrhosis), based on the phase 2 C-SALVAGE study.31 In this study, 79 patients, 66 of whom (84%) had previously been treated with a regimen containing a protease-inhibitor, were given 12 weeks of EBR/GZR and weight-based RBV. At entry, 34 patients (43%) harbored NS3A RASs, identified by population sequencing. Response rates were high, with 76/79 (96.2%) achieving SVR12, including 63/66 (95.5%) in patients with prior virologic failure, 31/34 (91.2%) in patients with baseline NS3 RASs, and 32/34 (94.1%) in cirrhotic patients. The presence of NS5A or dual NS3/NS5A RASs was associated with lower SVR12 rates (75% and 66%, respectively), but since only 3 patients had virologic failure, firm conclusions could not be drawn. The findings from the C-EDGE and C-SALVAGE trial led to the recommendation that RBV should be added to the combination of EBR/GZR and treatment should be extended to 16 weeks in GT1a patients with or without cirrhosis, if baseline RASs are detected (i.e. substitutions at amino acid positions 28, 30, 31 or 93).
Also, in GT1 patients with cirrhosis who are treatment-experienced with PEG-IFNα and RBV regimens, the addition of RBV can reduce the duration of retreatment when using LDV/SOF. The phase 2 SIRIUS trial32 compared 24 weeks of LDV/SOF with 12 weeks of LDV/SOF+RBV in treatment-experienced patients with GT1 and compensated cirrhosis. Both regimens had similar SVR12 rates, with 96% in the LDV/SOF+RBV group and 97% in the LDV/SOF alone group. This study’s findings increased options for GT1 treatment-experienced patients with cirrhosis by providing a regimen option with a shorter duration.
Augmenting response in treatment-experienced GT3 infection
In GT3 treatment-experienced patients, RBV is an important component of all currently recommended retreatment regimens. In a study by Planko et al.,33 96% (n = 26) of GT3 patients with compensated cirrhosis who were PEG-IFNα and RBV-experienced were successfully retreated with a 12-week duration of SOF/velpatasvir (VEL)+weight-based RBV, as compared to only 88% of those who received SOF/VEL alone (n = 26).
Treatment of GT4 infection
PEARL-I was a randomized, open label trial that evaluated the efficacy of paritaprevir, ombitasvir, and ritonavir (PrO) with or without RBV in treatment-naïve and treatment-experienced non-cirrhotic patients with GT4 infection.34 In 86 treatment-naïve patients, SVR12 rates were 100% (42/42) for the RBV-containing regimen and 90.9% (40/44) for the RBV-free regimen. There were 49 treatment-experienced patients who received the RBV-containing regimen, and all achieved SVR12. The PEARL-I trial demonstrated the efficacy of RBV for virologic cure in treatment-naïve and treatment-experienced GT4 patients.
Patients with decompensated cirrhosis
The ASTRAL-435 study was the first to compare the use of DAAs both with and without RBV in patients with decompensated cirrhosis and demonstrated the utility of RBV in achieving high SVR12 rates when combined with 12 weeks of SOF/VEL. Regardless of genotype, rates of viral response exceeded 85% when SOF/VEL was combined with RBV; this combination also resulted in the lowest rates of virologic failure for patients with GT3 (other studies have shown that SVR12 rates with GT3 are lower than rates for other genotypes).35 The SOF/VEL+RBV regimen was compared to SOF/VEL alone, each for 12 weeks, and SOF/VEL for 24 weeks. The response rate in the 12-week SOV/VEL+RBV arm was 94%, compared to 83% in SOF/VEL alone for 12 weeks and 86% in the SOF/VEL 24-week arm. Out of 267 patients treated in that study, 72 had pre-treatment NS5A RASs. Among these 72 patients, 64 (89%) had SVR12 compared to 169 of 183 patients (92%) who did not have pre-treatment NS5A RASs. In the SOF/VEL 24-week arm, the presence of NS5A RASs affected virologic cure, as the SVR12 among those with pre-treatment NS5A RASs was 90%, compared to a 98% SVR12 rate for those who had no pre-existing NS5A RASs. In addition, among the patients with GT1 who received SOF/VEL+RBV, the SVR12 rate was 100% for those who had NS5A RASs and 98% for those who did not, demonstrating that RBV is an important adjunct to increase the rate of virologic cure with a 12-week regimen in patients with baseline resistance. Based on the ASTRAL-4 study, the benefits of RBV in patients with decompensated cirrhosis include augmenting viral response, increasing the potency of HCV regimens in order to shorten treatment duration, and serving as a salvage treatment option for those patients in whom HCV treatment is of top priority in order to decrease risk of hepatocellular carcinoma and overall mortality.
Patients with liver transplants
For patients who are post-liver transplantation (with or without HCV treatment experience and/or compensated or decompensated cirrhosis) and infected with HCV 1, 4, 5 or 6, the only recommended, evidence-based regimen for treatment which includes RBV is in combination with LDV/SOF for 12 weeks. The evidence for use of RBV with LDV/SOF is provided by three studies. The SOLAR-136 study was a USA-based, phase 2, open-label study assessing LDV/SOF+RBV in patients with GT1 or 4 infection in two cohorts, one of which included patients who were post-liver transplant, with or without cirrhosis (Child-Pugh class A, B, or C). All of these patients were randomized to receive either 12 or 24 weeks of LDV/SOF with RBV. SVR12 rates among patients without cirrhosis and compensated cirrhosis were 96% and 98%, respectively. These rates were not affected by duration of treatment or cirrhosis status. In patients with Child-Pugh class B cirrhosis, the rates of SVR12 were similar with 12 weeks and 24 weeks of treatment (86% vs. 88%). Although there were lower SVR12 rates in patients with Child-Pugh class C disease (60% after 12 weeks of treatment and 75% after 24 weeks), this cohort was small (n = 9). Of note, 7 patients who had cirrhosis underwent liver transplant (4 before the end of treatment and 3 before post-treatment week 12). Six of 7 achieved SVR12; the seventh died of multiorgan failure and septic shock. Since SVR12 rates were similar despite duration of treatment (12 vs. 24 weeks) and only 4% of patients discontinued treatment due to adverse events from treatment drugs, this study led to the recommendation that 12 weeks of LDV/SOF+RBV is sufficient for patients after liver transplant. However, since all patients in the study received RBV, regardless of treatment duration, the role of RBV in higher SVR achievement could not be ascertained definitively from this study.
Second, the observational cohort HCV-TARGET37 included 347 patients with liver transplants, 279 of whom received LDV/SOF for 12 or 24 weeks. The SVR12 rates were 97% (152/157) for those who received RBV and 95% (116/122) in the group that did not take RBV. Patients who received RBV were more frequently GT1a, treatment-experienced, and had intact renal function. The rate of therapy discontinuation was only 1.3%. Third, a multicenter cohort38 of 162 patients (mostly GT1) assessed response rates of LDV/SOF with or without RBV for 8, 12 or 24 weeks. Overall, 94% and 98% achieved SVR12 when receiving LDV/SOF without or with RBV, respectively. The SVR12 rate among patients who received 8 weeks of treatment was 86%, but did not differ greatly for 12 versus 24 weeks (94% vs. 95%). SVR12 rates in the RBV groups for 12 and 24 weeks was 97% and 100%, respectively. These studies suggest that high rates of SVR can be achieved without RBV; however, the addition of RBV may be considered for patients with an unfavorable baseline profile (i.e. cirrhosis, treatment-experienced).
For patients with GT2 or GT3 infection (with or without HCV treatment experience and/or compensated cirrhosis), the ALLY-1 trial39 and that by Fontana et al.40 demonstrate that DCV/SOF+RBV for 12 weeks is an effective regimen. In the ALLY-1 trial,39 DCV/SOF+RBV was given for 12 weeks to 60 patients, both treatment-naïve and treatment-experienced, with Child-Pugh class A, B, or C cirrhosis after liver transplant. Eleven patients with GT3 were included in the trial; up to 83% (5/6) with advanced cirrhosis and 91% (10/11) with recurrent HCV after transplant achieved SVR12. In the study by Fontana et al.,40 the overall SVR12 rate with DCV/SOF+RBV (for all genotypes) was 91% (70/77). Only 3 patients had GT2 or GT3, and they all received DCV/SOF+RBV and achieved SVR12. There have been no studies in patients with decompensated cirrhosis after liver transplant, so the AASLD/IDSA guidelines4 recommend using DCV/SOF+RBV for 12 weeks or SOF/VEL with RBV for 12 weeks.
Resource-limited settings
More than 80% of the worldwide HCV burden is in low- and middle-income countries;41 however, treatment escalation in these areas has been hindered by the high cost of DAAs in developing countries.42 A study by Iyengar et al.43 analyzed and compared prices of DAAs in 30 countries, noting that people in developing countries had more risk of paying higher adjusted prices than people in developed countries. Furthermore, they noted the price of generic SOF was more than the annual earnings for individuals in 12 of the 30 countries they analyzed. The World Health Organization (commonly known as WHO) estimates that in Ukraine, for example, a 4-week course of PEG-IFNα and RBV is $139, compared to $812 for 4 weeks of LDV/SOF.44 These facts highlight the conundrum that although DAAs are more efficacious and have fewer side effects than PEG-IFNα /RBV regimens, the cost of DAAs remains prohibitive for most patients who need to be treated worldwide. Therefore, in resource-limited settings or countries with a low gross domestic product, RBV (in combination with PEG-IFNα) may be the only accessible treatment option at present based on cost, particularly for easier to treat genotypes.
Conclusions
The WHO has established a goal to treat 80% of those with chronic HCV worldwide by 2030, an aspiration that, with the advent of combination DAA-based regimens, appears within reach for the first time.45 With the recent changes in the guidelines and use of DAAs in the treatment of HCV, regimens have become more tolerable, effective, and less complicated, enabling HCV to be treated by primary care and midlevel providers,46 with minimal laboratory monitoring. Therefore, the use of RBV should be restricted to patients who have failed previous regimens and/or have decompensated cirrhosis, as there is data to support use in these populations. In both instances, as more and more new regimens become available, RBV’s utility will need to be revaluated, since RBV use is associated with significant anemia in decompensated patients47 and end-stage renal disease,48 and retreatment studies are relatively small and restricted to a few regimens. Addition of RBV to all DAA regimens to treat HCV patients is not advisable and may be hazardous. While there may be disagreement about RBV’s efficacy and incidence of adverse events in the current era of HCV treatment,49 we recommend that the use of RBV should be limited to treatment in complicated patients who are managed under close supervision and should not be used to treat patients in primary care settings with uncomplicated HCV.
Abbreviations
- DAAs
direct-acting antivirals
- DCV
daclatasvir
- DNA
deoxyribonucleic acid
- EBR
elbasvir
- FDA
Food and Drug Administration
- GT
genotype
- GZR
grazoprevir
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- LDV
ledipasvir
- PEG-IFNα
pegylated-interferon α
- PrO
paritaprevir, ritonavir, ombitasvir
- PrOD
paritaprevir, ritonavir, ombitasvir, dasabuvir
- RAS
resistance-associated substitutions
- RBV
ribavirin
- RNA
ribonucleic acid
- SOF
sofosbuvir
- SVR
sustained virologic response
- VEL
velpatasvir
- WHO
World Health Organization
References
- 1.Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vutien P, Jin M, Le MH, Nguyen P, Trinh S, Huang JF, et al. Regional differences in treatment rates for patients with chronic hepatitis C infection: Systematic review and meta-analysis. PLoS One. 2017;12:e0183851. doi: 10.1371/journal.pone.0183851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Present HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Available from: http://www.tasl.org.tr/upload/content/files/hcv-guidance-september-21-2017-d.pdf , accessed September 2017. [DOI] [PMC free article] [PubMed]
- 5.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 6.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 8.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 9.Testoni B, Levrero M, Durantel D. Mechanism of action of ribavirin in anti-HCV regimens: new insights for an age-old question? Gut. 2014;63:3–4. doi: 10.1136/gutjnl-2013-304528. [DOI] [PubMed] [Google Scholar]
- 10.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 11.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 12.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd J, Waugh N, Hewitson P. Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review. Health Technol Assess. 2000;4:1–67. [PubMed] [Google Scholar]
- 14.Di Bisceglie AM, Conjeevaram HS, Fried MW, Sallie R, Park Y, Yurdaydin C, et al. Ribavirin as therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Russmann S, Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem. 2006;13:3351–3357. doi: 10.2174/092986706778773059. [DOI] [PubMed] [Google Scholar]
- 16.REBETOL® (ribavirin USP) capsules, for oral use. Available from: http://www.merck.com/product/usa/pi_circulars/r/rebetol/rebetol_pi.pdf .
- 17.North Chicago, IL: Moderiba(R) [package insert]. Abbvie, Inc. Accessed February 2015. [Google Scholar]
- 18.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 20.Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631–640. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- 21.Meissner EG, Nelson A, Marti M, Masur H, Osinusi A, Kottilil S. Sustained virologic response for chronic hepatitis C infection after 27 days of treatment with sofosbuvir and ribavirin. Open Forum Infect Dis. 2014;1:013. doi: 10.1093/ofid/ofu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang L, Ward H, Kattakuzhy S, Wilson E, Kottilil S. Dual sofosbuvir and ribavirin therapy for chronic hepatitis C infection. Expert Rev Gastroenterol Hepatol. 2016;10:21–36. doi: 10.1586/17474124.2016.1119042. [DOI] [PubMed] [Google Scholar]
- 23.Pol S, Ghalib RH, Rustgi VK, Martorell C, Everson GT, Tatum HA, et al. Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis. 2012;12:671–677. doi: 10.1016/S1473-3099(12)70138-X. [DOI] [PubMed] [Google Scholar]
- 24.Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370:222–232. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
- 25.Suwanthawornkul T, Anothaisintawee T, Sobhonslidsuk A, Thakkinstian A, Teerawattananon Y. Efficacy of second generation direct-acting antiviral agents for treatment naïve hepatitis C genotype 1: A systematic review and network meta-analysis. PLoS One. 2015;10:e0145953. doi: 10.1371/journal.pone.0145953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 27.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 28.Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 29.Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: A randomized trial. Ann Intern Med. 2015;163:1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 30.Kwo P, Gane EJ, Peng CY, Pearlman B, Vierling JM, Serfaty L, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology. 2017;152:164–175.e4. doi: 10.1053/j.gastro.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Forns X, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, et al. Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent. J Hepatol. 2015;63:564–572. doi: 10.1016/j.jhep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Bourlière M, Bronowicki JP, de Ledinghen V, Hézode C, Zoulim F, Mathurin P, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS) Lancet Infect Dis. 2015;15:397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed] [Google Scholar]
- 33.Pianko S, Flamm SL, Shiffman ML, Kumar S, Strasser SI, Dore GJ, et al. Sofosbuvir plus velpatasvir combination therapy for treatment-experienced patients with genotype 1 or 3 hepatitis C virus infection: A randomized trial. Ann Intern Med. 2015;163:809–817. doi: 10.7326/M15-1014. [DOI] [PubMed] [Google Scholar]
- 34.Hézode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385:2502–2509. doi: 10.1016/S0140-6736(15)60159-3. [DOI] [PubMed] [Google Scholar]
- 35.Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 36.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS, Jr, Hassan MA, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017;66:1090–1101. doi: 10.1002/hep.29258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwok RM, Ahn J, Schiano TD, Te HS, Potosky DR, Tierney A, et al. Sofosbuvir plus ledispasvir for recurrent hepatitis C in liver transplant recipients. Liver Transpl. 2016;22:1536–1543. doi: 10.1002/lt.24614. [DOI] [PubMed] [Google Scholar]
- 39.Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493–1505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontana RJ, Brown RS, Jr, Moreno-Zamora A, Prieto M, Joshi S, Londoño MC, et al. Daclatasvir combined with sofosbuvir or simeprevir in liver transplant recipients with severe recurrent hepatitis C infection. Liver Transpl. 2016;22:446–458. doi: 10.1002/lt.24416. [DOI] [PubMed] [Google Scholar]
- 41.Suthar AB, Harries AD. A public health approach to hepatitis C control in low- and middle-income countries. PLoS Med. 2015;12:e1001795. doi: 10.1371/journal.pmed.1001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford N, Singh K, Cooke GS, Mills EJ, von Schoen-Angerer T, Kamarulzaman A, et al. Expanding access to treatment for hepatitis C in resource-limited settings: lessons from HIV/AIDS. Clin Infect Dis. 2012;54:1465–1472. doi: 10.1093/cid/cis227. [DOI] [PubMed] [Google Scholar]
- 43.Iyengar S, Tay-Teo K, Vogler S, Beyer P, Wiktor S, de Joncheere K, et al. Prices, costs, and affordability of new medicines for hepatitis C in 30 countries: An economic analysis. PLoS Med. 2016;13:e1002032. doi: 10.1371/journal.pmed.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guidelines for the screening care and treatment of persons with chronic hepatitis C infection: Updated version. Available from: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0093400/ [PubMed]
- 45.World Health Organization Combining hepatitis B. and C to reach elimination by 2030. Available from: http://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/ , accessed May 2016.
- 46.Kattakuzhy S, Gross C, Emmanuel B, Teferi G, Jenkins V, Silk R, et al. Expansion of treatment for hepatitis C virus infection by task shifting to community-based nonspecialist providers: A nonrandomized clinical trial. Ann Intern Med. 2017;167:311–318. doi: 10.7326/M17-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danish FA, Koul SS, Subhani FR, Rabbani AE, Yasmin S. Antiviral therapy in HCV-infected decompensated cirrhotics. Saudi J Gastroenterol. 2010;16:310–314. doi: 10.4103/1319-3767.70632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romero-Gómez M, Berenguer M, Molina E, Calleja JL. Management of anemia induced by triple therapy in patients with chronic hepatitis C: challenges, opportunities and recommendations. J Hepatol. 2013;59:1323–1330. doi: 10.1016/j.jhep.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Feld JJ, Jacobson IM, Sulkowski MS, Poordad F, Tatsch F, Pawlotsky JM. Ribavirin revisited in the era of direct-acting antiviral therapy for hepatitis C virus infection. Liver Int. 2017;37:5–18. doi: 10.1111/liv.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13:401–408. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- 51.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 52.Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100–2107. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- 53.Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804–811. doi: 10.1001/jama.2013.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]