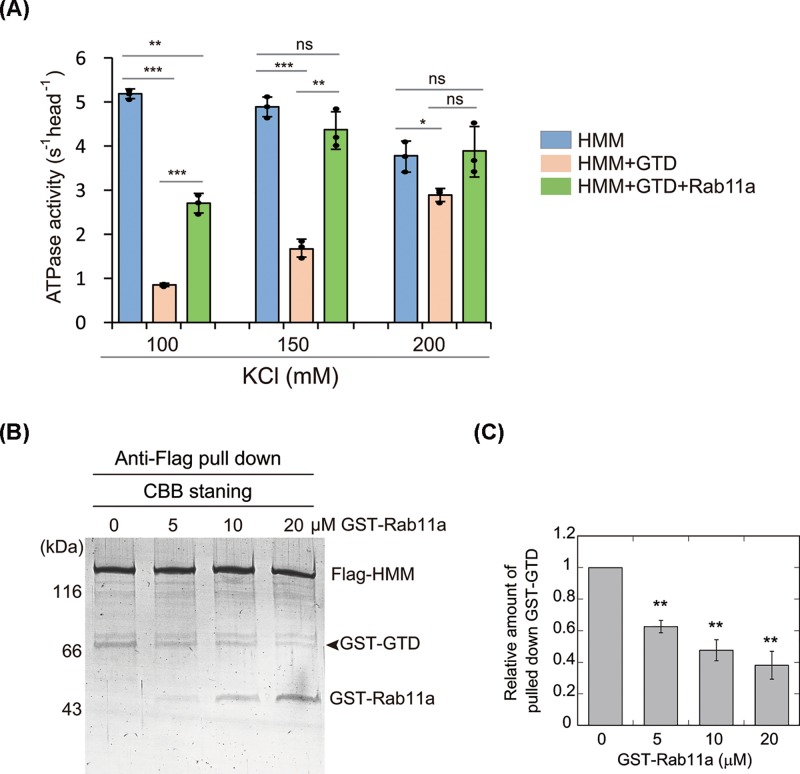
Figure 3. Rab11a activates Myo5b ATPase activity by weakening the head-GTD interaction of Myo5b.
(A) Rab11a reduces the inhibition of Myo5b–HMM ATPase activity by the GTD. The ATPase activity was measured in the presence of ∼80 nM Myo5b–HMM, 0–5 μM GTD, 0–12 μM GST–Rab11a, 40 μM actin in a solution containing 20 mM MOPS-KOH (pH 7.0), 100–200 mM KCl, 1 mM MgCl2, 1 mM DTT, 0.25 mg/ml BSA, 12 μM CaM, 0.5 mM ATP, 2.5 mM PEP, 20 U/ml pyruvate kinase and 1 mM EGTA. Values are mean ± S.D. from three independent assays. ns, no significance; *, P<0.05; **, P<0.01; ***, P<0.001. Panels (B) and (C) showing that Rab11a weakens the interaction between Myo5b–HMM and Myo5b–GTD. Flag-Myo5b-HMM (0.8 μM), GST-GTD (0.8 μM) and GST–Rab11a (0–20 μM) were pulled down by anti-Flag agarose, and the bound proteins were eluted by Flag peptide. The eluted proteins were subjected to SDS–PAGE and Commassie Blue staining. The remaining of GST–Rab11a in several pull down samples is due to incomplete washing of the beads (for detail, see ‘Materials and methods’ section). (B) SDS–PAGE representative of three independent assays. (C) The quantification of the pulled down GST–GTD (relative to that in the absence of GST–Rab11a). Values are the mean ± S.D. from three independent assays. ** (P<0.01) indicates significant difference compared to the first column.