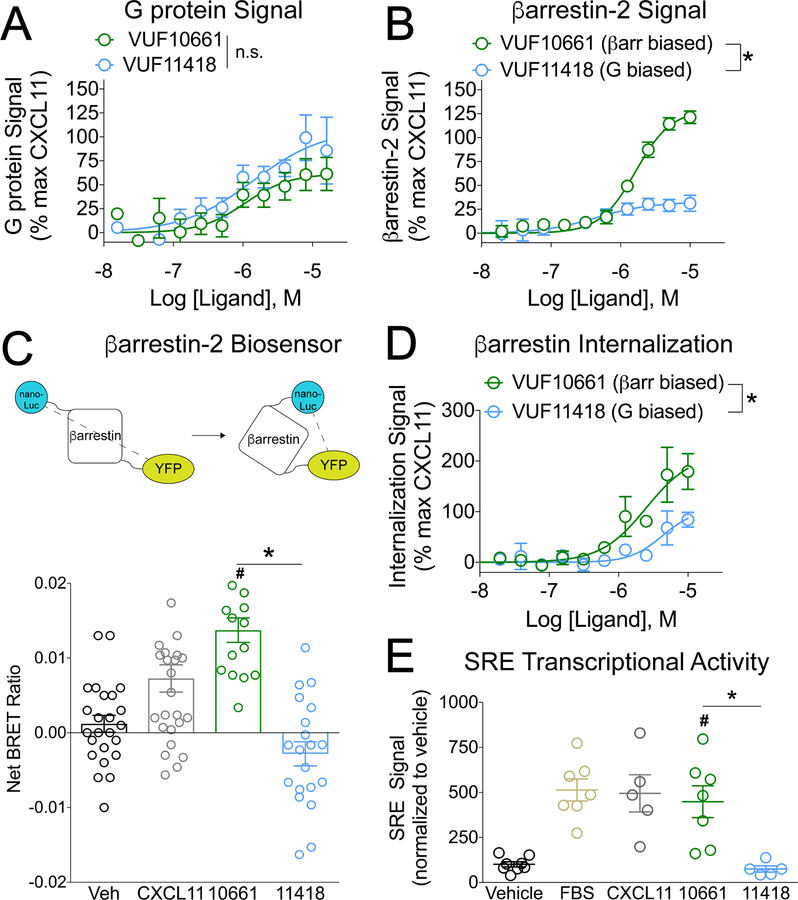
Fig 1. VUF10661 and VUF11418 are biased agonists of CXCR3.
(A) Transfected HEK 293T “ΔG six” cells expressing CXCR3 and the interrogated Gα subunit were analyzed for Gαi activation by the TGF-α shedding assay as described in Materials and Methods. The cells were treated for 1 hour with vehicle or the indicated concentrations of the G protein–biased CXCR3 agonist VUF11418 or the β-arrestin–biased CXCR3 agonist VUF10661. The extent of G protein signal is expressed as a percentage of that induced by the CXCR3 agonist CXCL11. Data are means ± SEM of eight or nine experiments. (B) Transfected HEK 293T cells expressing CXCR3-Rluc and β-arrestin2-YFP were analyzed for β-arrestin2 recruitment by BRET as described in Materials and Methods. The cells were treated for 5 min with vehicle or the indicated concentrations of VUF11418 or VUF10661. The extent of β-arrestin2 recruitment is expressed as a percentage of that induced by the CXCR3 agonist CXCL11, which was used as a control. Data are means ± SEM of four experiments. (C) Top: Schematic of the detection of a conformational change in β-arrestin by measuring BRET between a nanoLuc donor and a YFP acceptor. Bottom: Transfected HEK 293T cells expressing CXCR3 and the β-arrestin biosensor were analyzed by BRET to detect changes in the conformation of β-arrestin. The cells were treated for five minutes with vehicle, 1 μM VUF11418, 1 μM VUF10661, or 250 nM CXCL11 as a positive control and the net BRET ratio was calculated by subtracting the vehicle signal from the drug signal. Data are from 15 to 26 wells from four independent experiments. (D) U2OS cells stably expressing β-arrestin2–dependent internalization assay components (enzyme fragments tagged to β-arrestin2 and endosomes) and transiently expressing CXCR3 were analyzed for CXCR3 internalization as described in Materials and Methods. The cells were treated for 90 minutes with vehicle or the indicated concentrations of VUF11418 or VUF10661. As a control, the cells were treated with CXCL11 (1 μM). The percentage of CXCR3 internalization was calculated relative to that induced by CXCL11. Data are means ± SEM of three to five experiments. (E) Transfected HEK 293T cells expressing CXCR3 and a serum response element (SRE) reporter were analyzed by luminescence to measure changes SRE transcriptional activation. The cells were treated for 5 hours with vehicle, 10 μM VUF11418, 10 μM VUF10661, 10% FBS, or 1 μM CXCL11. The SRE signal was normalized to that of the vehicle control. Data are means ± SEM of five to eight experiments. For (A), (B), and (E), data were analyzed by two-way ANOVA; *P < 0.05 when comparing VUF10661 to VUF11418. For (C) and (F), data were analyzed by one-way ANOVA and Tukey post hoc analysis. *P < 0.05 when comparing VUF10661 to VUF11418; #P < 0.05 when compared to vehicle.