Figure 7.
Passage of Fibroblast-Free Alveolospheres as Single-Cell Suspensions
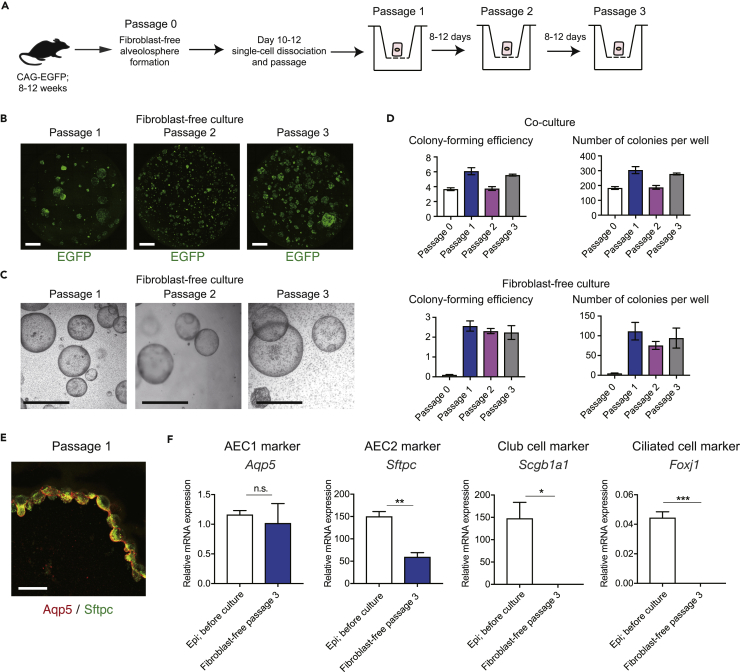
(A) Experimental scheme of fibroblast-free alveolosphere passaging. Colonies were dissociated and 2 × 103–5 × 103 cells were plated.
(B) Confocal micrographs of representative wells of passaged spheres (epithelial cells; EGFP; entire Transwell stored in 24-well plates is shown) after 8–12 days.
(C) Bright-field images of representative wells of passaged spheres.
(D) Colony-forming efficiency of fibroblast-free and co-cultured alveolospheres. To calculate colony-forming efficiency, all colonies formed in each well were counted and the number of colonies was divided by 5 × 103 (for primary cultures and for passaging of co-cultured alveolospheres) or 2 × 103–5 × 103 (for passaging of fibroblast-free alveolospheres). Colony-forming efficiency of primary fibroblast-free alveolosphere culture (P0) was 0.1% ± 0.02 (5.0 ± 0.9 spheres per well). Fibroblast-free alveolospheres were passaged at least three times without loss of colony-forming efficiency. For P0, n = 12 wells for fibroblast-free culture and n = 9 wells for co-culture pooled from at least two independent cell sorting; for P1, P2, or P3, n = 3 or 4 wells for fibroblast-free culture and n = 6 wells for co-culture pooled from at least two independent passaging procedures.
(E) Representative section of passaged fibroblast-free alveolosphere (passage 1) on day 8 labeled with antibodies against Aqp5/Sftpc.
(F) qPCR analysis of AEC1, AEC2, and non-alveolar epithelial markers using cells obtained from passaged spheres (passage 3, day 8). Data represent mean ± SEM (n = 3 wells for cultured cells and n = 3 animals for sorted cells) and are representative of two independent experiments. mRNA levels were normalized to that of Actb. *p < 0.05, **p < 0.01, ***p < 0.001 (unpaired Student's t test [two-tailed]).
Scale bar: 1 mm (B and C), 25 μm (E).