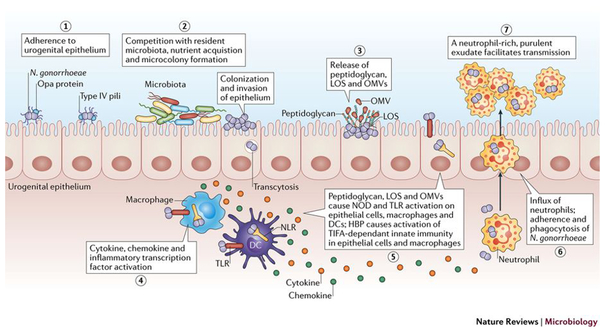
Figure 1: Overview of Neisseria gonorrhoeae infection.
During initial infection, N. gonorrhoeae adheres to host epithelial cells through Type IV pili (step 1), which retract and enable epithelial interactions with other prominent surface structures160,161. After initial adherence, N. gonorrhoeae replicates and forms microcolonies (step 2), and possibly biofilms34,49, and likely competes with the resident microbiota. When colonizing the epithelium, N. gonorrhoeae is capable of invasion and transcytosis. During these initial stages in infection, N. gonorrhoeae releases fragments of peptidoglycan, lipooligosaccharide (LOS), and outer membrane vesicles(OMVs)111,162,163 (step 3) that activate Toll-like receptor (TLR) and nucleotide-binding oligomerization domain-like receptor (NOD) signaling in epithelial cells, macrophages, and dendritic cells (DCs)111,164,165. NOD and TLR signaling from these cells leads to activation of inflammatory transcription factors and the release of cytokines and chemokines (step 4). N. gonorrhoeae also releases heptose-1,7-bisphosphate (HBP) that activates TRAF-interacting protein with forkhead-associated domain (TIFA) immunity115 (step 5). The release of pro-inflammatory cytokines and chemokines by these innate immune signaling pathways creates cytokine and chemokine gradients that recruit large numbers of polymorphonuclear leukocytes (PMNs) neutrophils to the site of infection (step 6), where they interact with and phagocytose N. gonorrhoeae. The influx of neutrophils comprises a purulent exudate that then facilitates tranmission (step 7).