Abstract
Efficient and site-specific chemical modification of proteins under physiological conditions remains a challenge. Here we report that 1,4-dinitroimidazoles are highly efficient bifunctional bioconjugation reagents for protein functionalization and peptide macrocyclization. Under acidic to neutral aqueous conditions, 1,4-dinitroimidazoles react specifically with cysteines via a cine-substitution mechanism, providing rapid, stable and chemoselective protein bioconjugation. On the other hand, although unreactive towards amine groups under neutral aqueous conditions, 1,4-dinitroimidazoles react with lysines in organic solvents in the presence of base through a ring-opening & ring-close mechanism. The resulting cysteine- and lysine-(4-nitroimidazole) linkages exhibit stability superior to that of commonly employed maleimide-thiol conjugates. We demonstrate that 1,4-dinitroimidazoles can be applied in site-specific protein bioconjugation with functionalities such as fluorophores and bioactive peptides. Furthermore, a bisfunctional 1,4-dinitroimidazole derivative provides facile access to peptide macrocycles by crosslinking a pair of cysteine or lysine residues, including bicyclic peptides of complex architectures through highly controlled consecutive peptide macrocyclization.
The selective formation of protein bioconjugates under physiological conditions is a challenging task. Here, the authors report that 1,4-dinitroimidazoles are reagents of choice for protein bioconjugation at either cysteine or lysine sites within short times and provide facile access to peptide macrocycles.
Introduction
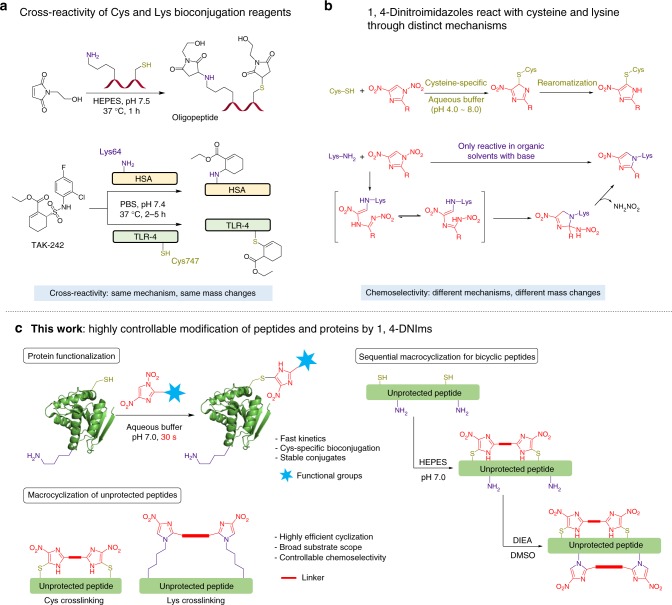
Chemical modification of proteins has the potential to expand their function by mimicking naturally occurring post-translational modifications1–3. Methods for site-specific modification of proteins are invaluable in generating biological therapeutic agents, such as antibody-drug conjugates (ADCs) and PEGylated biologics3. Although biorthogonal methods using engineered amino acids provide an elegant strategy for protein functionalization in complex biological mixtures4, chemical means to prepare homogeneous proteins with defined modification in sufficient quantities are still required. Amongst all natural amino acids, cysteines are the most important amino acid for protein modification due to their intrinsic nucleophilicity3,5. Methodologies for cysteine modification often rely on electrophilic reagents such as maleimides (Michael acceptors) and aryl and alkyl halides3,6. Recently, a number of strategies have been developed to expand the toolbox for cysteine modification, including transition metal-mediated bioconjugation7, a cysteine-to-dehydroalanine conversion strategy8,9, enzymatic click cysteine ligation10, and strain-release alkylation11, among others5,6,12–14. Notwithstanding their advantages for bioconjugation, cysteines are rare in human proteins (1.9% of residues) and often exist as disulfides, and therefore unavailable for modification. Lysine residues, on the other hand, occur much more commonly in proteins (5.9% of residues in human proteins) and are generally exposed on the protein surface, providing a convenient target for bioconjugation. Typical lysine bioconjugation methods also rely on nucleophilic addition by lysine ε-amine, such as acylation, activated esters15,16, and the recently developed arylation and condensation reactions17–20. Despite recent advances in Cys and Lys bioconjugation, chemoselective modifications are often difficult to achieve in a proteinaceous environment. For example, maleimides are the most widely used cysteine bioconjugation reagents21. However, maleimides often react with N-terminal amine and lysine under neutral pH, resulting in a mixture of Cys and Lys adducts (Fig. 1a)14. Compound TAK-242 is employed as a covalent Lys inhibitor to modify Lys64 of human serum albumin selectively; however, TAK-242 was found to react with Cys747 of Toll-like receptor-4 via the same nucleophilic addition mechanism (Fig. 1a). Moreover, the cross-reactivity of these bioconjugation reagents always causes the same mass change to target proteins, posing additional challenges for comprehensive proteomic studies. Although a certain degree of chemoselectivity between Cys and Lys can be achieved by careful control of reaction conditions (such as fine-tuning the pH of the media), cross-reactivity remains a major challenge for most bioconjugation reagents.
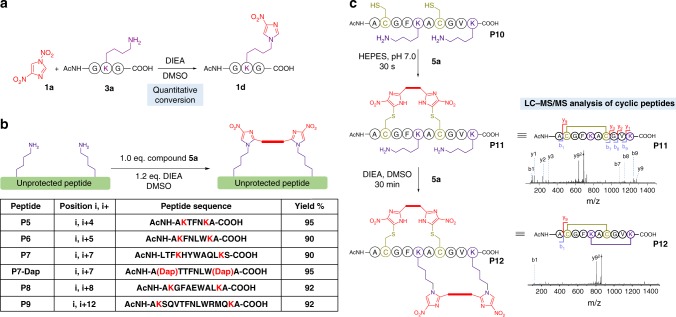
Fig. 1.
1,4-Dinitroimidazoles as bifunctional bioconjugation reagents. a Cross-reactivity of cysteine and lysine modification reagents. b 1,4-DNIms react with cysteine and lysine through two distinct mechanisms and display different chemoselectivity under aqueous and organic solvents. c Application of 1,4-DNIms in protein functionalization and peptide macrocyclization
The stability of protein conjugates is another key criterion for bioconjugation reagents. Maleimides are the most widely used cysteine bioconjugation reagents, and are currently employed in most of the ADCs in the market and in clinical trials21. However, the susceptibility of the thiosuccinimidyl linkage to hydrolysis and thiol exchange reactions has limited its application in biological systems22–24. Efforts to overcome this limitation have been made by developing maleimide derivatives that lead to stable bioconjugates, such as the exocyclic olefinic maleimides, pyridazinediones, and hydrolysable maleimide derivatives24–29. Therefore, alternative bioconjugation reagents with high reactivity and selectivity are still in need to generate stable protein conjugates for pharmaceutical applications. Herein, we report that 1,4-dinitroimidazoles (1,4-DNIms) are a class of rapid, chemoselective, and bifunctional bioconjugation reagents for protein functionalization and peptide macrocyclization. 1,4-DNIms are cysteine-specific under a wide pH range (pH 3.0–8.0) in aqueous solutions, and modify cysteine residues in proteins quantitatively in seconds through a cine-substitution mechanism (Fig. 1b). Although unreactive towards lysines under neutral aqueous conditions, 1,4-DNIms modify lysine residues efficiently in organic solvents, such as dimethyl sulfoxide (DMSO), with weak bases through an ring-opening and ring-closing mechanism for protein bioconjugation. The resulting cysteine- and lysine-(4-nitroimidazole) linkages are significantly more stable than the commonly used maleimide conjugates. Using this chemistry, we have achieved cysteine-specific functionalization of proteins with fluorophores and bioactive peptides. In addition, we demonstrate that bisfunctional 1,4-DNIm compounds can provide facile access to peptide macrocycles with complex ring structures.
Results
1,4-DNIms are rapid and Cys-specific bioconjugation reagents
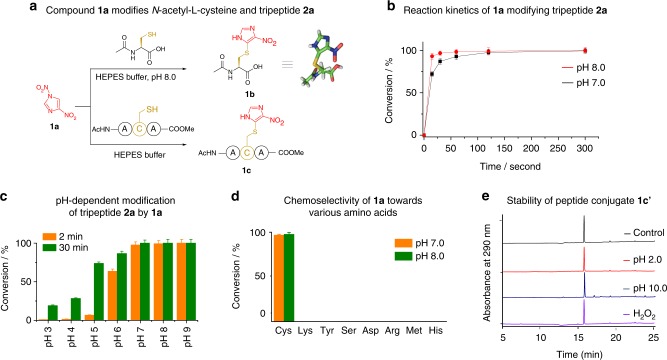
To initiate our investigation, we synthesized compound 1a and sought to evaluate its potential for cysteine bioconjugation (Fig. 2a). Incubation of compound 1a (1.0 mM) with N-acetyl-l-cysteine (1.0 mM) in 100 mM HEPES buffer at pH 8.0 leads to rapid and quantitative conversion to a cysteine adduct 1b in 15 s (Fig. 2a). Structural characterization of adduct 1b by nuclear magnetic resonance (NMR) and X-ray crystallographic analysis indicate that the cysteine thiol is directly conjugated to C-5 of the nitroimidazole (Fig. 2a, Supplementary Fig. 1). This result suggests that the reaction proceeds through a cine-substitution mechanism with N1-nitro as the leaving group upon nucleophilic addition of thiol to C-5 of compound 1a, which is followed by re-aromatization (Fig. 2a, Supplementary Fig. 2). With C-5 hindered by methyl substitution, 5-methyl-1,4-nitroimidazole 2c was unreactive with cysteine and remained intact under assay conditions, supporting this reaction mechanism (Supplementary Fig. 3).
Fig. 2.
Compound 1a modifies cysteine thiol with high efficiency and specificity by generating stable conjugates. a Reaction of compound 1a with cysteine and tripeptide 2a. The CCDC reference number of compound 1b is 1860451. b Reaction kinetics of compound 1a (0.2 mM) with tripeptide 2a (0.1 mM) at pH 7.0 and 8.0. c The reaction between of 1a (0.2 mM) and 2a (0.1 mM) under various pH for 2 min and 30 min. d Reactions of compound 1a (0.2 mM) with nucleophilic amino acids (0.1 mM) for 15 min under pH 7.0 and 8.0. e Chemical stability of the tripeptide-(4-nitroimidazole) conjugate 1c' at pH 2.0, pH 10.0, and under oxidative conditions (10 mM H2O2 in Na2CO3 buffer at pH 8.0) at 37 °C for 10 h. The conversion values represent means ± SDs (standard deviations) of three independent experiments
The high reactivity of compound 1a prompted us to investigate its ability to modify cysteine residues in peptides. Tripeptide 2a was therefore synthesized and treated with compound 1a in HEPES buffer at pH 7.0. High-performance liquid chromatography (HPLC) analysis showed that 92% conversion of peptide 2a was achieved within 1 min, and full conversion was achieved after 5 min by generating peptide conjugate 1c (Fig. 2b, Supplementary Fig. 4). The reaction was significantly accelerated by elevating the pH of the buffer to 8.0, leading to full conversion in only 15 s (Fig. 2b, Supplementary Fig. 5). We next evaluated the pH dependence of this reaction. After incubating 1,4-DNIm 1a with tripeptide 2a at pH 7.0–9.0 for 2 min, near-quantitative modification was observed (Fig. 2c, Supplementary Figs. 4-6). Prolonging the incubation time to 30 min led to 28% and 74% conversion of tripeptide 2a even under acidic conditions at pH 4.0 and 5.0, respectively, demonstrating the wide applicable pH range of this reaction (Fig. 2c, Supplementary Figs. 7–10).
Next, we evaluated the chemoselectivity of compound 1a under aqueous conditions. Lysines are rich in natural proteins and usually solvent-exposed on protein surface, therefore representing a major competition. In a competitive assay, incubation of tripeptide 2a (0.1 mM) and a lysine-containing tripeptide 3a (1.0 mM) with compound 1a (0.12 mM) in HEPES buffer at pH 7.0 yielded conjugate 1c near quantitatively (Supplementary Fig. 11). No lysine adduct of tripeptide 3a was detected by liquid chromatography–mass spectrometry (LC-MS) analysis, demonstrating the dominating selectivity of 1,4-DNIms towards cysteine thiol over lysine ε-amine. Furthermore, compound 1a did not react with nucleophilic amino acids, including Tyr, Ser, Asp, Arg, Met, and His, under neutral aqueous conditions, further establishing the thiol specificity of 1,4-DNIms (Fig. 2d). Perfluoroaryl compounds have shown great potential in cysteine bioconjugation of peptides13. To compare their efficiency with 1,4-DNIms, we chose 6,6′-sulfonylbis(1,2,3,4,5-pentafluorobenzene) (pf1), the most reactive perfluoroaryl reagent reported so far30, as the model compound. First, we observed that compound pf1 exhibited a very low solubility (<0.05 mM) in aqueous solutions, whereas 1,4-DNIm 1a has a solubility higher than 10 mM. In HEPES buffer, pH 7.4, (pf1) at a concentration of 0.04 mM was unreactive with peptide 2a, whereas 1,4-DNIm 1a modified (2a) near quantitatively (Supplementary Fig. 12). This result shows that under neutral aqueous conditions, 1,4-DNIms are superior to perfluoroaryl compounds in terms of solubility and reactivity towards cysteine residues. Together, under acidic to neutral aqueous conditions, 1,4-DNIms are highly efficient and cysteine-specific bioconjugation reagents.
The stability of protein conjugates is a major concern for bioconjugation reagents. Therefore, we evaluated the stability of 1,4-DNIm 1a and the resulting (4-nitroimidazole)-thiol products. HPLC analysis showed that N-phenyl maleimide underwent complete hydrolysis in 2 h in 100 mM phosphate-buffered saline (PBS) buffer at pH 7.4, yielding the maleamic acid (Supplementary Fig. 13). In contrast, compound 1a displayed resistance to hydrolysis, remaining unchanged for up to 10 h (Supplementary Fig. 14). Next, we investigated the stability of the (4-nitroimidazole)-thiol linkage. Thiol-maleimide and thiol-arylation conjugates are reported to degrade under acidic, basic, or oxidative conditions25,30,31. We incubated conjugate 1c' in aqueous buffer at pH 2.0, pH 10.0, or in the presence of 10 mM H2O2 for 10 h at 37 °C. HPLC analysis indicated that conjugate 1c' did not decompose under tested conditions (Fig. 2e, Supplementary Fig. 15), whereas the N-ethyl maleimide conjugate of 2a′ underwent complete hydrolysis at pH 10.0 (Supplementary Fig. 16). Together, both 1,4-DNIm 1a and its cysteine conjugate exhibited enhanced stability compared with maleimides and maleimide-cysteine conjugates under various conditions, offering an attractive alternative for cysteine bioconjugation of proteins.
Protein conjugates bearing different classes of functionalities, such as fluorophores and biomolecules, are valuable in the fields of biomaterials and chemical biology. To test the applicability of 1,4-DNIms in protein modification and their compatibility with diverse functional components, we synthesized fluorescent 7-nitrobenzo-Z-oxa-1,3 diazole (NBD)-conjugate 4a and peptide arginine-glycine-aspartic acid (RGD)-conjugate 4b bearing a 1,4-DNIm warhead for cysteine bioconjugation (Fig. 3a). Sortase A (SrtA) with one free cysteine (Cys192) and 26 lysines was selected as a model protein. It was expected that 1,4-DNIms could achieve cysteine-specific labeling by overcoming the challenge of a large number of surface-exposed Lys under neutral aqueous conditions. Incubation of SrtA (20 µM) with compound 4a (200 µM) for 1 h in PBS buffer, pH 7.0, resulted in quantitative conversion to NBD-modified SrtA, as determined by LC-MS analysis (Fig. 3c). The NBD-modified SrtA was further digested by trypsin and subjected to tandem mass spectrometry (MS) analysis. Results showed that the SrtA186–205 segment carried the NBD modification at the Cys192 residue (Supplementary Fig. 17). To further confirm the Cys-specific labeling, SrtA was pre-treated with iodoacetamide (IAA) before incubation with NBD-(1,4-DNIm) 4a. No fluorescent labeling was observed for the IAA-treated SrtA, further indicating that 1,4-DNIms selectively modified cysteine residues (Fig. 3d). RGD peptides bind to the integrin receptors of cells and are widely used for targeted delivery of chemotherapeutics32,33. Compound 4b with a 1,4-DNIm moiety at the N terminus of RGD sequence was then incubated with SrtA under neutral buffer. To our delight, full conversion to RGD-SrtA conjugate was observed after 1 h (Fig. 3c). These results demonstrate that 1,4-DNIms are compatible with diverse functional components during protein modification.
Fig. 3.
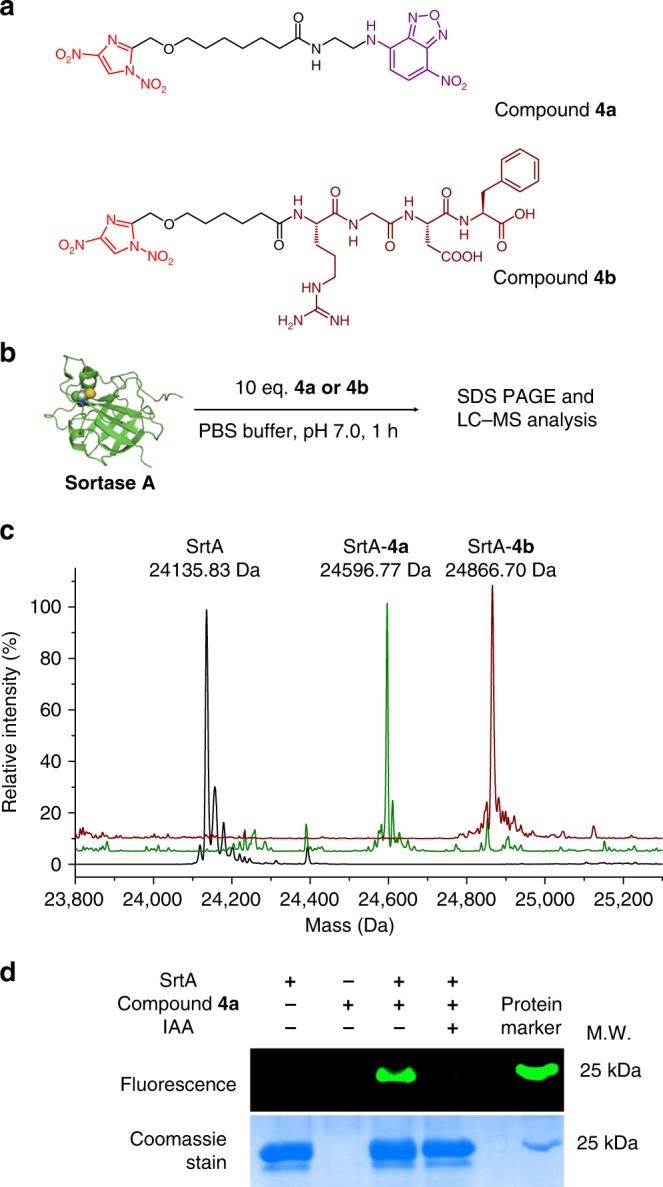
Modification of SrtA with 1,4-DNIms bearing a fluorescent NBD moiety and an RGD peptide. a Chemical structures of 1,4-DNIm derivatives 4a and 4b. b Bioconjugation of SrtA by 1,4-DNIm derivatives. Conditions: SrtA (20 µM), compound 4a or 4b (200 µM), 100 mM PBS buffer, pH 7.0, 1 h. c LC-MS analysis of SrtA modified by compounds 4a and 4b. Molecular mass (average): SrtA calcd. 24,136.03 Da, observed 24,135.83 Da; (SrtA-4a) conjugate calcd. 24,596.44 Da, observed 24,596.77 Da; (SrtA-4b) conjugate calcd. 24,866.77 Da, observed 24,866.70 Da. d SDS-PAGE analysis of the fluorescent labeling of SrtA or iodoacetamide-treated SrtA by compound 4a
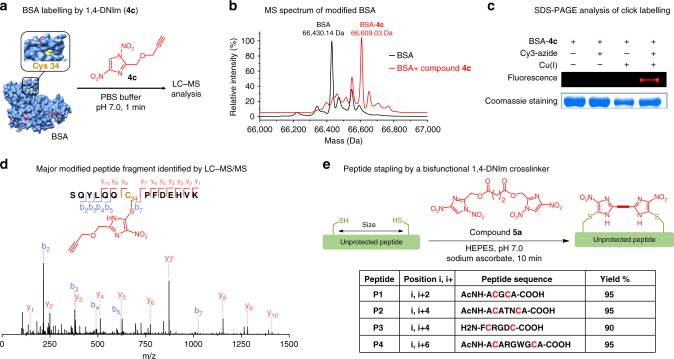
To make 1,4-DNIms as a general approach to prepare diverse protein conjugates, we synthesized compound 4c bearing an alkyne group for further functionalization through 1,3 dipolar azide–alkyne cycloaddition (AAC) (Fig. 4a). Bovine serum albumin (BSA), with a single free cysteine (Cys34) and 59 lysine residues, was chosen as a model protein. Treatment of BSA (100 µM) with compound 4c (500 µM) led to quantitative modification in 1 min, as determined by LC-MS analysis (Fig. 4b). To identify the modification site, the resulting BSA-4c conjugate was reduced, digested by trypsin, and subjected to LC-MS/MS analysis. Results showed that the modification occurs exclusively at the Cys34 residue (Fig. 4d), and no lysine modification is detected. Subsequently, fluorescent labeling of the BSA-4c conjugate was realized with a fluorogenic azide, sulfo-Cy3-azide, through CuAAC reaction. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed that BSA was successfully labeled with fluorophore Cy3 through the alkyne handle of compound 4c as control reactions without copper or Cy3-azide failed to produce any observable fluorescence (Fig. 4c). Therefore, the successful incorporation of an alkyne group as the click reaction handle through 1,4-DNIm bioconjugation provides a facile method for cysteine-selective addition of various payloads to proteins of interest.
Fig. 4.
Thiol-specific labeling of BSA protein and peptide stapling by 1,4-DNIm derivatives. a Bioconjugation of derivative 4c with BSA. The free thiol of Cys34 residue is highlighted in yellow. b ESI-MS spectra of BSA protein before and after treatment with compound 4c. Molecular mass (average): BSA calcd. 66,429.98 Da, observed 66,430.14 Da; BSA-4c calcd. 66,609.12 Da, observed 66,609.03 Da. c Gel image of the reaction between BSA-4c conjugate and sulfo-Cy3-azide by a CuAAC reaction. d LC-MS/MS analysis of a modified peptide fragment from trypsin digestion of BSA-4c conjugate. e Macrocyclization of cysteine-containing unprotected peptides by a bisfunctional 1,4-DNIm (5a). Conditions: peptides (1.0 mM) and compound 5a (1.0 mM) and sodium ascorbate (2.0 mM) were incubated in 100 mM HEPES buffer at pH 7.0 for 10 min before acidified with formic acid and subjected to HPLC analysis
The potential toxicity of the thiol-(4-nitroimidazole) linkage generated by bioconjugation is an important concern for the biological application of the 1,4-DNIm chemistry. To address this issue, we synthesized a fluorescein isothiocyanate (FITC)-labeled RGD peptide and an (1,4-DNIm)-modified cyclic RGD (cRGD) peptide (Supplementary Fig. 18). At a concentration of 10 µM, RGD peptides were efficiently uptaken by 293T cells, as demonstrated by the cell staining assay of FITC-RGD. Upon incubation with 10 µM cRGD-(4-nitroimidazole) conjugate, the viability of 293T cells was not affected (Supplementary Fig. 18), indicating that the thiol-(4-nitroimidazole) is not toxic under assay conditions.
Cys-specific peptide stapling by bisfunctional 1,4-DNIms
Cyclic peptides have great potential as therapeutic agents and biological tools, and thus the development of stapling reagents is of particular interest13,18,34–37. The capability of 1,4-DNIms in selective cysteine modification prompted us to explore their application in peptide stapling. Thus, a bisfunctional 1,4-DNIm (5a) was synthesized and incubated with unprotected peptides containing two cysteine residues at varying sites under neutral aqueous buffer for 10 min. Sodium ascorbate was added to the reaction to avoid disulfide formation caused by air oxidation. High conversions were observed for all peptides examined by yielding corresponding peptide macrocycles, as determined by HPLC analysis (Fig. 4e). In particular, Cys-specific crosslinking of peptide P3 with unprotected N terminus was achieved, demonstrating the chemoselectivity of 1,4-DNIms towards thiol groups over N-terminal amine.
1,4-DNIms modify lysine amine in organic solvents
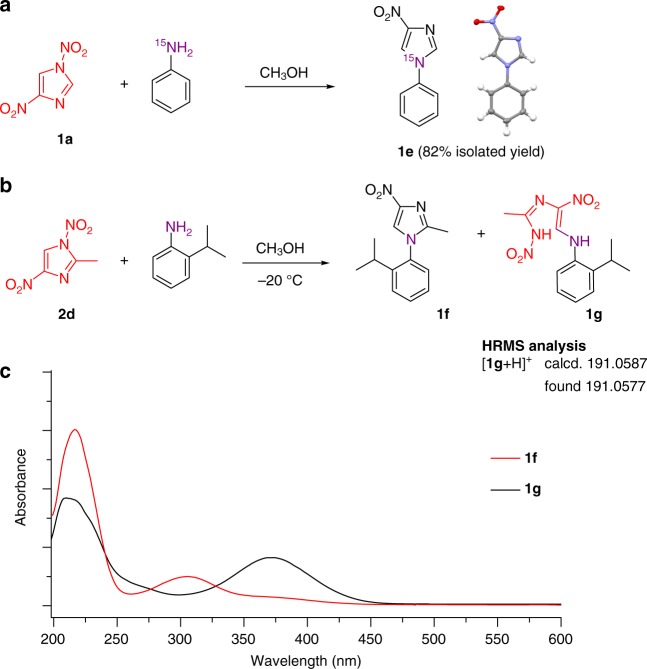
Although lysines and N-terminal amine groups in proteins are inert to 1,4-DNIm treatment under neutral aqueous conditions, reactions between amines and 1,4-DNIms have been reported in the synthesis of nitroimidazole derivatives in organic solvents. Early evidence and recent computational studies suggest that the reaction is initiated by nucleophilic attack at C5 position of 1,4-DNIms and follows an ANRORC-like mechanism, consisting of the addition of a nucleophile followed by ring-opening and ring closure step (Fig. 1b)38–40. To provide mechanistic insight of this reaction, we utilized 15N-labeled aniline to react with 1,4-DNIm (1a), generating product 1e in 82% (Fig. 5a). High-resolution MS (HRMS) analysis showed that (1e) is isotopically labeled, indicating that the 15N atom from aniline is present in the product (Fig. 5a). NMR characterization of (1e) by analyzing 15N-C and 15N-H coupling signals further indicated that the 15N of analine was indeed incorporated into the newly formed imidazole ring by forming two C-15N bonds with C2 and C5 of (1a) (Supplementary Figs. 19–24). Furthermore, X-ray crystallographic analysis unambiguously confirmed the structure of (1e) (Fig. 5a).
Fig. 5.
Mechanistic investigation of the reactions between 1,4-DNIms and amines. a 1,4-DNIm 1a reacts with 15N-labeled aniline to produce (1e). The CCDC reference number of compound 1e is 1876959. HRMS (ESI) analysis of product (1e): m/z calcd. for C9H8N215NO2 [M + H]+ 191.0587, found 191.0577; m/z calcd. for C9H7N215NNaO2 [M + Na]+ 213.0406, found 213.0400. b 2-isopropylaninline reacts with 2-methyl-1,4-DNIm 2d. HRMS (ESI) analysis of intermediate (1g): m/z calcd. for C13H17N5NaO4 [M + Na]+ 330.1178, found 330.1172; c UV–Vis characterization of products 1f and 1g
To examine whether the reaction is initiated by nucleophilic attack at the C5 positions of 1,4-DNIms, we employed 4-(2-aminoethyl)phenol to react with compound 1a, 5-methyl-1,4-DNIm 2c and 2-methyl-1,4-DNIm 2d (Supplementary Fig. 25). Results showed that compounds 1a and 2d reacted with similar efficiency, leading to 52% and 50% conversion, respectively. In contrast, 5-methyl-1,4-DNIm 2c was inert under assay conditions (2% conversion). No accumulation of ring-opening intermediates was observed in these reactions. Such substitution effect supports C5 position as the site for nucleophilic addition by amines.
To examine the proposed ring-opening step, we chose 2-isopropylaninline and 2-methyl-1,4-DNIm (2d) as reacting partners (Fig. 5b), in which the bulky substitutions are expected to slow down the ring closure and facilitate the capture of ring-opening intermediates. To our delight, in addition to the final product 1f, the accumulation of a labile intermediate 1g was observed by thin layer chromatography (Supplementary Fig. 26). HRMS analysis of the reaction mixture also revealed an intermediate with a mass matching (1g) (Fig. 5c). The ultraviolet–visible spectra of product 1g display a characteristic absorbance at 369 nm (Fig. 5c), suggesting the presence of an extended π-conjugation resulted from the opening of imidazole ring. Furthermore, gas chromatography-MS analysis of intermediate (1g) yielded molecular fragments that match the proposed structure of (1g) (Supplementary Fig. 27). Although we were not able to perform NMR analysis of (1g) due to its spontaneous conversion to product 1f, our data support the proposal that 1,4-DNIms react with amines through the ANRORC-like mechanism.
Next, we explored the application of 1,4-DNIms in Lys bioconjugation of peptides. After screening various reaction conditions, we found that in DMSO with DIEA (N,N-diisopropylethylamine) added as the base, compound 1a (0.1 mM) was able to modify a lysine-containing tripeptide 3a (0.1 mM) with near-quantitative conversion in 30 min (Fig. 6a). HRMS analysis and NMR analysis determined that product 1d is a ε-(4-nitroimidazole)-Lys derivative (Fig. 6a, Supplementary Figs. 28–32). Next, we explored the chemoselectivity of compound 1a in DMSO via incubation with nucleophilic amino acids, including Tyr, Ser, Trp, His, Asp, and Arg. No reaction occurred with these amino acids, indicating that this reaction was lysine-specific. In comparison with perfluoroaryl reagent pf1, 1,4-DNIm 1a displayed similar reactivity when modifying a lysine-containing tripeptide in DMSO (Supplementary Fig. 33). The 4-nitroimidazole linkage resulted from Lys-(1,4-DNIm) bioconjugation was highly stable and remained intact after incubation under acidic (pH 2.0), basic (pH 10.0), and oxidative (10 mM H2O2) conditions at 37 °C for 10 h (Supplementary Fig. 34). To sum up, 1,4-DNIms react specifically with cysteine thiols under neutral aqueous conditions, and modify lysine ε-amines through a distinct mechanism with high selectivity in organic solvents in the absence of thiols by generating stable conjugates.
Fig. 6.
Macrocyclization of peptides by a bisfunctional 1,4-DNIm through lysine and cysteine crosslinking. a Lysine modification by 1,4-DNIm 1a. b Peptide macrocyclization by bisfunctional 1,4-DNIm 5a. c Preparation of a bicyclic peptide by controllable sequential macrocyclization by 1,4-DNIm 5a
Lys-specific peptide stapling by bisfunctional 1,4-DNIms
Next, we applied bisfunctional 1,4-DNIm 5a in peptide macrocyclization by crosslinking two lysine residues. By varying the site of lysine residues (i,i + 4 to i,i + 12), a series of peptide macrocycles were prepared in high yields (Fig. 6b). Diaminopropionic acid (Dap) in peptide P7-Dap was also efficiently crosslinked, demonstrating the versatility of this protocol. Peptide macrocyclization usually stabilizes the secondary structures of peptides and improves their proteolytic stability. Indeed, circular dichroism analysis showed that cyclization of peptide P8 increased the content of α-helical structure (Supplementary Fig. 35). Regarding the proteolytic stability of peptides, linear peptide P7 was completely hydrolyzed in the presence of proteinase K in 30 min, whereas cyclized P7 was significantly more stable and remained intact for up to 4 h (Supplementary Fig. 36).
Preparation of bicyclic peptides by bisfunctional 1,4-DNIms
The synthesis of peptide architectures with overlapping rings is particularly challenging because it often requires appropriate orthogonal ring-closing reactions, as well as the incorporation of functionalized unnatural amino acids41,42. The controllable chemoselectivity of 1,4-DNIms under aqueous and organic solvents provides a convenient method to integrate two consecutive stapling reactions and to enable regioselective construction of complex bicyclic peptides. For this purpose, we synthesized a model peptide P10 containing two cysteine and two lysine residues for i,i + 5-position macrocyclization (Fig. 6c). Incubation of stapling reagent 5a (0.1 mM) with peptide P10 (0.1 mM) in 100 mM HEPES buffer at pH 7.0 resulted in quantitative conversion to the cyclic product P11 in 30 s as determined by LC-MS (Supplementary Fig. 37). The Cys-specific crosslinking are confirmed by the lack of fragmentation between two cysteine residues during tandem MS analysis (Fig. 6c, Supplementary Fig. 38). The reaction mixture of 5a and P10 was then lyophilized without purification, redissovled in DMSO, and treated with another 2 equiv compound 5a. Results showed that a second crosslinking between lysine residues in the monocyclic peptide P11 was achieved in 30 min by yielding a bicyclic peptide P12 with overlapping ring structures, as indicated by tandem MS analysis (Fig. 6c, Supplementary Fig. 39). Thus, bisfunctional 1,4-DNIms are powerful peptide stapling reagents that allow controllable consecutive peptide stapling to generate peptide scaffolds with entangled macrocycles.
Discussion
In summary, we have demonstrated that 1,4-DNIms are highly efficient, bifunctional bioconjugation reagents that provide stable protein conjugates and peptide macrocycles. This chemistry allows facile incorporation of various functional groups into proteins at defined positions. Importantly, 1,4-DNIms represent a class of electrophilic bioconjugation reagents that react with cysteine and lysine through completely different mechanisms. Consequently, 1,4-DNIms display vastly different reactivity and chemoselectivity under different reaction conditions, making them highly controllable in protein modification without cross-reactivity. In view of the kinetics, selectivity, and stability of the 1,4-DNIm bioconjugation reactions, 1,4-DNIm compounds are a valuable addition to the current toolbox for peptide and protein bioconjugation.
Methods
General information
All the reagents and solvents were obtained from Sigma-Aldrich, Alfa-Aesar, or Acros, and used directly without further purification. Amino acids and derivatives were obtained from commercial sources. BSA was purchased from BBI Life Sciences and used without further purification. SrtA 5M was a kind gift from Prof. Yi Cao at the Nanjing University. NMR spectra were recorded on Bruker AMX-400 instrument for 1H NMR at 400 MHz and 13C NMR at 100 MHz, using tetramethylsilane as an internal standard. The following abbreviations (or combinations thereof) were used to explain multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and br = broad. Coupling constants, J, are reported in Hertz units (Hz). HRMS were recorded on an Agilent Mass spectrometer using ESI-TOF (electrospray ionization-time of flight). HPLC profiles were obtained on Agilent 1260 HPLC system using commercially available columns.
Modification of peptides and proteins by 1,4-DNIms
Typically, peptide (0.1 mM) was incubated with 1,4-DNIm (0.1 mM) and in HEPES buffer (100 mM) at indicated pH at 25 °C for indicated time before analyzis by HPLC and MS. For protein modification, 20 µM protein was incubated with 10 eq. of 1,4-DNIm derivative in PBS buffer at pH 7.0 at 25 °C for 1 h. The reaction mixture was quenched by the addition of 1% HCOOH or removing the excess compound by PD10 desalting column before subjecting to LC-MS analysis.
Fluorescent labeling of BSA via CuAAC reaction
BSA-4c conjugate was prepared by incubating 15 µM BSA and 5 eq. compound 4c in 100 mM PBS buffer at pH 7.0. The excess compound 4c was then removed by PD10 desalting column, and the BSA-4c conjugate was eluted with 100 mM PBS buffer, pH 7.2. BSA-4c conjugate (15 μM) was further incubated with 100 μM sulfo-Cy3-azide, 250 μM CuSO4, 500 μM BTTAA (bis[(tertbutyltriazoyl)methyl]-[(2-carboxymethyltriazoyl)methyl]amine), and 2.5 mM freshly prepared sodium ascorbate for 1 h at 25 ℃ before quenching with 5 mM BCA. The reaction samples were analyzed by SDS-PAGE gels and imaged by Gel DocTM XR+ (Bio-Rad). The protein gels were also stained by Coomassie Brilliant Blue.
Supplementary information
Acknowledgements
This work is supported by the 1000-Youth Talents Plan, NSF of China (Grant 21778030), NSF of Jiangsu Province (Grant BK20160640), and the Fundamental Research Funds for the Central Universities (Grants 14380138 and 14380131). We thank Prof. Yi Cao (Nanjing University) for the gift of SrtA protein and Prof. Qi Zhang (Fudan University) for helpful discussion during the preparation of the manuscript.
Author contributions
Q.L. designed and carried out the chemical synthesis and reactions. Y.T. performed most of the biochemical experiments regarding protein modifications. W.S. performed the cell culture, staining experiments, and the cellular toxicity assay. L.J. supported biochemical experiments. H.W. designed and supervised the project. All authors discussed the results and commented on the manuscript. Q.L., Y.T., and H.W. wrote the manuscript. All authors have given approval to the final version of the manuscript.
Data availability
The X-ray crystallographic coordinates for structures of compounds 1b and 1e have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers of 1860451 and 1876959, respectively. All relevant data are available in supplementary information and from the authors.
Competing interests
All authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Glenn Burley and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-08010-2.
References
- 1.Walsh CT, Garneau-Tsodikova S, Gatto GJ., Jr. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl. 2005;44:7342–7372. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- 2.Davis BG. Biochemistry. Mimicking posttranslational modifications of proteins. Science. 2004;303:480–482. doi: 10.1126/science.1093449. [DOI] [PubMed] [Google Scholar]
- 3.Boutureira O, Bernardes GJ. Advances in chemical protein modification. Chem. Rev. 2015;115:2174–2195. doi: 10.1021/cr500399p. [DOI] [PubMed] [Google Scholar]
- 4.Krall N, da Cruz FP, Boutureira O, Bernardes GJL. Site-selective protein-modification chemistry for basic biology and drug development. Nat. Chem. 2016;8:102–112. doi: 10.1038/nchem.2393. [DOI] [PubMed] [Google Scholar]
- 5.deGruyter JN, Malins LR, Baran PS. Residue-specific peptide modification: a chemist’s guide. Biochemistry. 2017;56:3863–3873. doi: 10.1021/acs.biochem.7b00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, et al. Pi-Clamp-mediated cysteine conjugation. Nat. Chem. 2016;8:120–128. doi: 10.1038/nchem.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinogradova EV, Zhang C, Spokoyny AM, Pentelute BL, Buchwald SL. Organometallic palladium reagents for cysteine bioconjugation. Nature. 2015;526:687–691. doi: 10.1038/nature15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright TH, et al. Posttranslational mutagenesis: a chemical strategy for exploring protein side-chain diversity. Science. 2016;354:597. doi: 10.1126/science.aag1465. [DOI] [PubMed] [Google Scholar]
- 9.Chalker JM, Lercher L, Rose NR, Schofield CJ, Davis BG. Conversion of cysteine into dehydroalanine enables access to synthetic histones bearing diverse post-translational modifications. Angew. Chem. Int. Ed. Engl. 2012;51:1835–1839. doi: 10.1002/anie.201106432. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Spokoyny AM, Zou Y, Simon MD, Pentelute BL. Enzymatic “click” ligation: selective cysteine modification in polypeptides enabled by promiscuous glutathione S-transferase. Angew. Chem. Int. Ed. Engl. 2013;52:14001–14005. doi: 10.1002/anie.201306430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianatassio R, et al. Organic chemistry. Strain-Release amination. Sci. 2016;351:241–246. doi: 10.1126/science.aad6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vara, B. A. et al. Scalable thioarylation of unprotected peptides and biomolecules under Ni/photoredox catalysis. Chem. Sci.10.1039/c1037sc04292b (2018). [DOI] [PMC free article] [PubMed]
- 13.Spokoyny AM, et al. A perfluoroaryl-cysteine S(N)Ar chemistry approach to unprotected peptide stapling. J. Am. Chem. Soc. 2013;135:5946–5949. doi: 10.1021/ja400119t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Thiol specific and tracelessly removable bioconjugation via michael addition to 5-methylene pyrrolones. J. Am. Chem. Soc. 2017;139:6146–6151. doi: 10.1021/jacs.7b00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Muthoosamy K, Pfisterer A, Neumann B, Weil T. Site-selective lysine modification of native proteins and peptides via kinetically controlled labeling. Bioconjug. Chem. 2012;23:500–508. doi: 10.1021/bc200556n. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Moreira V, Alegre-Requena JV, Herrera RP, Marzo I, Gimeno MC. Synthesis of luminescent squaramide monoesters: cytotoxicity and cell imaging studies in HeLa cells. RSC Adv. 2016;6:14171–14177. doi: 10.1039/C5RA24521D. [DOI] [Google Scholar]
- 17.Tanaka K, Fujii Y, Fukase K. Site-selective and nondestructive protein labeling through azaelectrocyclization-induced cascade reactions. Chembiochem. 2008;9:2392–2397. doi: 10.1002/cbic.200800336. [DOI] [PubMed] [Google Scholar]
- 18.Bandyopadhyay A, Gao J. Iminoboronate-based peptide cyclization that responds to pH, oxidation, and small molecule modulators. J. Am. Chem. Soc. 2016;138:2098–2101. doi: 10.1021/jacs.5b12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HG, Lautrette G, Pentelute BL, Buchwald SL. Palladium-mediated arylation of lysine in unprotected peptides. Angew. Chem. Int. Ed. Engl. 2017;56:3177–3181. doi: 10.1002/anie.201611202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tung CL, Wong CTT, Fung EYM, Li XC. Traceless and chemoselective amine bioconjugation via phthalimidine formation in native protein modification. Org. Lett. 2016;18:2600–2603. doi: 10.1021/acs.orglett.6b00983. [DOI] [PubMed] [Google Scholar]
- 21.Jain N, Smith SW, Ghone S, Tomczuk B. Current ADC linker chemistry. Pharm. Res. 2015;32:3526–3540. doi: 10.1007/s11095-015-1657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toda N, Asano S, Barbas CF., III Rapid, stable, chemoselective labeling of thiols with Julia-Kocienski-like reagents: a serum-stable alternative to maleimide-based protein conjugation. Angew. Chem. Int. Ed. Engl. 2013;52:12592–12596. doi: 10.1002/anie.201306241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin AD, Kiick KL. Tunable degradation of maleimide-thiol adducts in reducing environments. Bioconjug. Chem. 2011;22:1946–1953. doi: 10.1021/bc200148v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyon RP, et al. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nat. Biotechnol. 2014;32:1059–1062. doi: 10.1038/nbt.2968. [DOI] [PubMed] [Google Scholar]
- 25.Kalia D, Pawar SP, Thopate JS. Stable and rapid thiol bioconjugation by light-triggered thiomaleimide ring hydrolysis. Angew. Chem. Int. Ed. Engl. 2017;56:1885. doi: 10.1002/anie.201609733. [DOI] [PubMed] [Google Scholar]
- 26.Kalia D, Malekar PV, Parthasarathy M. Exocyclic olefinic maleimides: synthesis and application for stable and thiol-selective bioconjugation. Angew. Chem. Int. Ed. Engl. 2016;55:1432–1435. doi: 10.1002/anie.201508118. [DOI] [PubMed] [Google Scholar]
- 27.Forte N, et al. Tuning the hydrolytic stability of next generation maleimide cross-linkers enables access to albumin-antibody fragment conjugates and tri-scFvs. Bioconjug. Chem. 2018;29:486–492. doi: 10.1021/acs.bioconjchem.7b00795. [DOI] [PubMed] [Google Scholar]
- 28.Lee MTW, Maruani A, Baker JR, Caddick S, Chudasama V. Next-generation disulfide stapling: reduction and functional re-bridging all in one. Chem. Sci. 2016;7:799–802. doi: 10.1039/C5SC02666K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruani A, et al. A plug-and-play approach to antibody-based therapeutics via a chemoselective dual click strategy. Nat. Commun. 2015;6:6645. doi: 10.1038/ncomms7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lautrette G, Touti F, Lee HG, Dai P, Pentelute BL. Nitrogen arylation for macrocyclization of unprotected peptides. J. Am. Chem. Soc. 2016;138:8340–8343. doi: 10.1021/jacs.6b03757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontaine SD, Reid R, Robinson L, Ashley GW, Santi DV. Long-term stabilization of maleimide-thiol conjugates. Bioconjug. Chem. 2015;26:145–152. doi: 10.1021/bc5005262. [DOI] [PubMed] [Google Scholar]
- 32.Nieberler, M. et al. Exploring the role of RGD-recognizing integrins in cancer.Cancers (Basel)9, 10.3390/cancers9090116 (2017). [DOI] [PMC free article] [PubMed]
- 33.Haubner R, et al. Cyclic RGD peptides containing beta-turn mimetics. J. Am. Chem. Soc. 1996;118:7881–7891. doi: 10.1021/ja9608757. [DOI] [Google Scholar]
- 34.Gongora-Benitez M, Tulla-Puche J, Albericio F. Multifaceted roles of disulfide bonds. Peptides as therapeutics. Chem. Rev. 2014;114:901–926. doi: 10.1021/cr400031z. [DOI] [PubMed] [Google Scholar]
- 35.Verdine GL, Hilinski GJ. Stapled peptides for intracellular drug targets. Methods Enzymol. 2012;503:3–33. doi: 10.1016/B978-0-12-396962-0.00001-X. [DOI] [PubMed] [Google Scholar]
- 36.Frost JR, Scully CC, Yudin AK. Oxadiazole grafts in peptide macrocycles. Nat. Chem. 2016;8:1105–1111. doi: 10.1038/nchem.2636. [DOI] [PubMed] [Google Scholar]
- 37.White CJ, Yudin AK. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011;3:509–524. doi: 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
- 38.Suwinski J, Szczepankiewicz W, Holt EM. Reactions of 1,4-dinitroimidazoles with hydrazines. Tetrahedron. 1996;52:14905–14916. doi: 10.1016/0040-4020(96)00903-9. [DOI] [Google Scholar]
- 39.Plas HCVd. The SN(ANRORC) mechanism: a new mechanism for nucleophilic substitution. Acc. Chem. Res. 1978;11:462. doi: 10.1021/ar50132a005. [DOI] [Google Scholar]
- 40.Gallardo-Fuentes S, Contreras R, Ormazábal-Toledo R. Origins of the ANRORC reactivity in nitroimidazole derivatives. RSC Adv. 2016;6:25215–25221. doi: 10.1039/C6RA00199H. [DOI] [Google Scholar]
- 41.Cromm, P. M. et al. Orthogonal ring-closing alkyne and olefin metathesis for the synthesis of small GTPase-targeting bicyclic peptides. Nat. Commun. 7, 10.1038/ncomms11300 (2016). [DOI] [PMC free article] [PubMed]
- 42.Bird GH, et al. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc. Nat. Acad. Sci. USA. 2010;107:14093–14098. doi: 10.1073/pnas.1002713107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures of compounds 1b and 1e have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers of 1860451 and 1876959, respectively. All relevant data are available in supplementary information and from the authors.