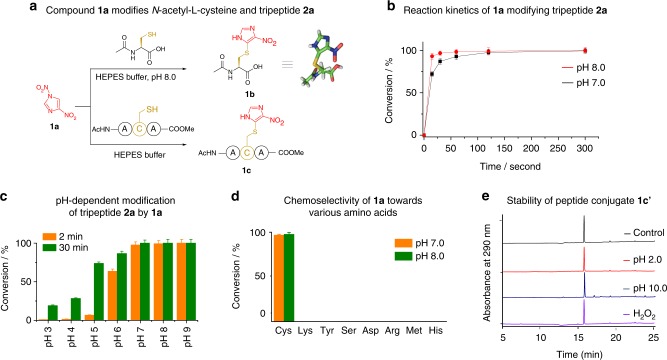
Fig. 2.
Compound 1a modifies cysteine thiol with high efficiency and specificity by generating stable conjugates. a Reaction of compound 1a with cysteine and tripeptide 2a. The CCDC reference number of compound 1b is 1860451. b Reaction kinetics of compound 1a (0.2 mM) with tripeptide 2a (0.1 mM) at pH 7.0 and 8.0. c The reaction between of 1a (0.2 mM) and 2a (0.1 mM) under various pH for 2 min and 30 min. d Reactions of compound 1a (0.2 mM) with nucleophilic amino acids (0.1 mM) for 15 min under pH 7.0 and 8.0. e Chemical stability of the tripeptide-(4-nitroimidazole) conjugate 1c' at pH 2.0, pH 10.0, and under oxidative conditions (10 mM H2O2 in Na2CO3 buffer at pH 8.0) at 37 °C for 10 h. The conversion values represent means ± SDs (standard deviations) of three independent experiments