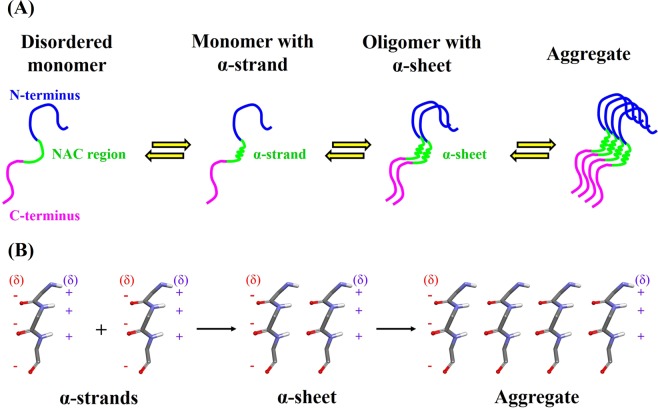
Figure 6.
Probable role of α-strand in the aggregation of α-synuclein. (A) Suggested stages of aggregation process. N-terminus, NAC region and C-terminus are highlighted in blue, green and pink, respectively. Biophysical studies have confirmed that isolated α-synuclein monomer is intrinsically disordered in vitro and aggregation results in the formation of amyloid fibrils. Occasionally, region 72–74 of α-synuclein monomer adopts an α-strand conformation. The α-strands of α-synuclein oligomers interact through hydrogen bonding to form α-sheet structure. Subsequently, α-sheet acts as a possible nucleus that initiate aggregation and fibrillation of α-synuclein. (B) A model for α-sheet intermediate where partial charges on the interface are indicated by red (negative charges) and blue (positive charges) colors.