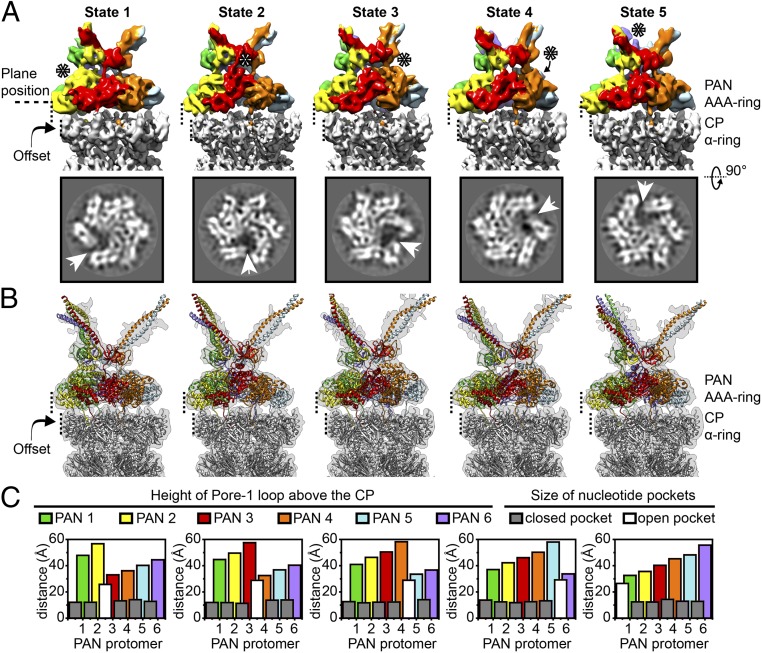
Fig. 2.
The PAN-proteasome exists in five rotated spiral-staircase conformations. (A) In the row above, density maps of AAAob from the five conformational states are displayed. For a fixed position of the PAN offset on the CP, the states differ in conformation of their AAA ring, and inclination of the N domain (OB ring and coiled coils). PAN is colored according to protomer, and in each state, the protomer occupying the highest position is indicated by asterisks. AAAob densities are placed in the context of the respective CP (in gray) to emphasize the fixed position of the offset. In the row below, slices through the indicated plane position are displayed. The existence of a split site is clearly visible in the slices and is further highlighted by white arrowheads. (B) Models of different states of the PAN-proteasome are superimposed on the respective PSC densities. In the models, PAN is colored according to protomer, and the CP is in gray. (C) Plot of pore loop height and nucleotide pocket depth for each conformational state (SI Appendix, SI Materials and Methods). Height of the tip of pore helices (α5 helix from each PAN protomer) above the plane of the CP is plotted for every PAN protomer, and is colored coherently. The size of a nucleotide pocket is plotted as an overlay. In all of the states, there is a distinct open pocket (colored white) between the highest and lowest pore helices, while the other pockets (closed) are of similar size, and colored gray.