Fig. 2. Confocal laser scanning microscopic images of H2O2-treated biofilms.
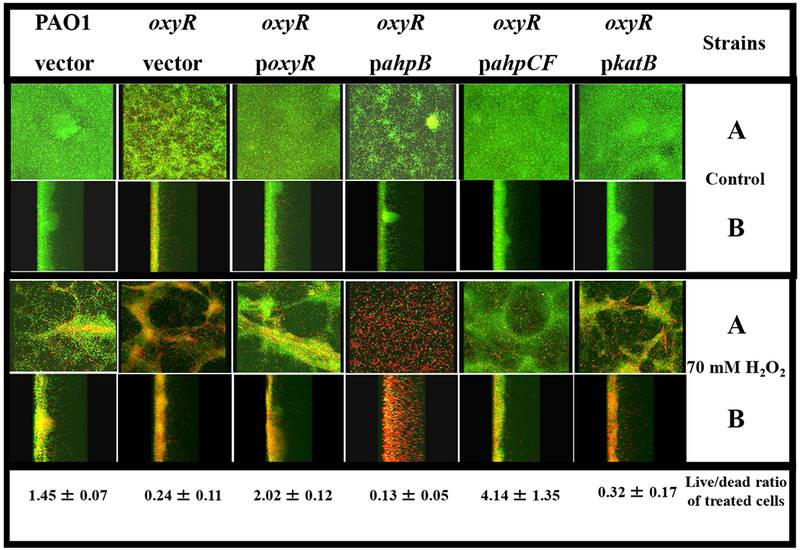
Biofilms were grown in confocal “friendly” flow chambers as previously described by Lequette and Greenberg [30]. Briefly, bacteria were grown aerobically in L-broth at 37°C until the stationary growth phase, diluted 1:50 into 1% TSB and a 0.2 ml suspension used to inoculate flow cells (Stovall Life Sciences, Inc., Greensboro, NC). Flow was initiated at a rate of 0.17 ml min−1 after the bacteria were allowed to attach for 1 hr. After a 3 day incubation at room temperature (~23°C), biofilms were treated with 70 mM H2O2 for 30 min. The biofilms were then stained with a live/dead viability stain composed of SYTO 9 and propidium iodine (Molecular Probes, Inc., Eugene, OR). Biofilm images were obtained using an LSM 510 confocal microscope (Carl Zeiss, Inc., Germany). The excitation and emission wavelengths for green fluorescence were 488 nm and 500 nm, while those for red fluorescence were 490 nm and 635 nm, respectively. All biofilm experiments were repeated at least 3 times. The live/dead ratios of the biofilms were calculated using the 3D for LSM (V.1.4.2) software (Carl Zeiss, Inc., Germany). The panels labeled A are top/bottom images while those in B are saggital images. The top third panel depicts the strains used in these studies. The live to dead ratio of treated bacteria is given on the bottom panel for each strain.
