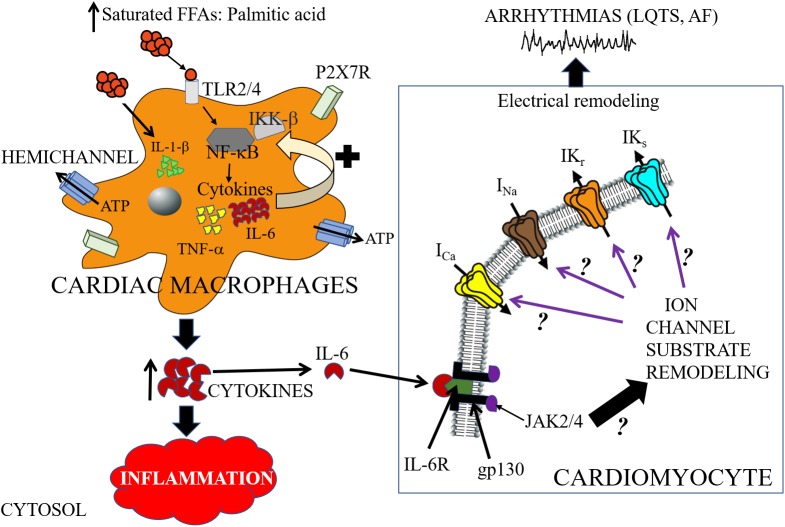
FIGURE 3.
Cartoon depiction illustrating potential saturated free-fatty acid mechanisms that may underlie arrhythmogenic events. High-fat diet induce elevations of saturated free-fatty acids (SFFAs) leading to increased expression of proinflammatory cytokines in macrophages. Cardiac macrophages sense high concentrations of SFFAs through the toll-like receptor 2 (TLR2) and/or toll-like receptor 4 (TLR4) which induces expression of inflammatory target genes including tumor necrosis factor (TNF)-α and interleukin-6 (IL-6), by regulating the activities of nuclear factor-κB (NF-κB) (Youssef-Elabd et al., 2012). SFFAs also induce interleukin-1β secretion via the NLRP3 inflammasome in macrophages, which is independent of the TLR4 pathway (Henao-Mejia et al., 2012). Hemichannels control the exchange of small molecules including ATP (Willebrords et al., 2016). The released ATP binds to the cell-surface purinergic receptors P2X7R, and can control inflammation by regulating the release of pro-inflammatory cytokines (including IL-1β) (Gicquel et al., 2017). There is a paucity of lipid studies involving the IL-6 signaling pathway, and therefore the molecular mechanisms of IL-6 modulation of cardiac function is poorly understood. In cardiomyocytes, IL-6 mediates its effect through its classical pathway. IL-6 binds to the glycoprotein 80 transmembrane IL-6 receptor (IL-6R) (Yamasaki et al., 1988), which leads to dimerization and activation of the signal transducing protein of glycoprotein 130 (gp130) and its downstream effector signaling molecules Janus kinase (JAK) 2/4 (Ihle, 1995; Heinrich et al., 2003; Drucker et al., 2010). We hypothesize that SFFA mediated cardiac electrical remodeling that increase vulnerability to arrythmias may occur through SFFAs/TLR/NF-kB-IL-6 mediated altered gene and protein expression of ion channel subunits, trafficking and/or gating defects. Distinguishing among these signaling pathways is likely to provide mechanistic insights that will influence considerations of anti-cytokine therapy for the management of metabolic disorders and arrythmias. The black ? indicates that whether or how activation of JAK2/4 leads to remodeling of ion channel molecular partners is poorly understood. The black? (associated with the purple arrows), represents an unresolved role for IL-6 modulation of major cardiac ion channels (ICa, INa, IKr, and IKs) in lipotoxicity and pathogenesis of arrhythmias including long QT syndrome (LQTS) and atrial fibrillation (AF).