Abstract
Involvement of multiple physiological pathways and complex pathogenesis is responsible for the onset and progression of type 2 diabetes mellitus (T2DM). Since it is difficult to manage multiple pathophysiological defects by monotherapy, a combination therapy with two or more oral antidiabetic agents (OADs) may help achieve euglycemia in T2DM patients. Choice of OADs is difficult with growing armamentarium of antidiabetic therapy. Ideally, drug combination should aim at reversal of known pathogenic abnormalities and demonstrate improvement in the overall metabolic health rather than simply reduce glycosylated hemoglobin (HbA1c) levels. Increased glucose reabsorption, a faulty pathological mechanism, is targeted by a novel class of drugs, namely, the sodium-glucose cotransporter-2 (SGLT2) inhibitors. Combination of SGLT2 inhibitors and other OADs complement each other due to their unique mechanism of action. In addition, the glucose-lowering effect of SGLT2 inhibitors remains independent of β-cell function and insulin sensitivity which reduces the chances of severe hypoglycemia in patients receiving these agents. Clinical studies from the past favor the use of SGLT2 inhibitors in combination with other agents to achieve better HbA1c levels, weight loss, and blood pressure control. In this review, we have made an attempt to explore the recommended guidelines for combination therapy, its advantages as either combination therapy or fixed-dose combinations therapy, and the role of SGLT2 inhibitors as a choice of drug as a combination with other OADs.
Keywords: Combination therapy, glucose-lowering effect, oral antidiabetic drugs, sodium-glucose cotransporter-2 inhibitors, type 2 diabetes mellitus
INTRODUCTION
According to the World Health Organization, the prevalence of type 2 diabetes mellitus (T2DM) has almost quadrupled from the year 1980 to 2014 and was accountable for over 1.5 million deaths worldwide in the year 2012.[1] Involvement of multiple physiological pathways and complex pathogenesis of diabetes explains the multifaceted morbidity noted in individuals with T2DM.[2]
The physiological defects that occur during the onset and progression of T2DM include impaired function of eight organ systems; these are collectively called as the “Ominous Octet.”[2,3] These include inappropriate or suboptimal function of the muscles, liver, β-cells, adipocytes (increased rate of lipolysis), gastrointestinal tract (deficiency or resistance of the hormone incretin), α-cells (excess glucagon secretion), kidneys (increased glucose reabsorption), and brain (resistance to insulin and dysregulation of neurotransmitter).[2]
As multiple pathophysiological defects cannot be managed by monotherapy, there is a need for combination therapy to target all pathophysiological pathways of T2DM and euglycemia.[3] It is also important that the drug combination should aim at reversal of known pathogenic abnormalities and demonstrate improvement in the overall metabolic health rather than simply reduce glycosylated hemoglobin (HbA1c) levels.[3]
The choice of glucose-lowering agent must be made carefully, particularly when a diverse range of pharmacological agents (consisting of at least 12 drug classes) are available for the treatment of T2DM.[4] Of these, biguanides (metformin), sulfonylureas (SUs), meglitinides, dipeptidyl peptidase-4 inhibitors (DPP-4i), thiazolidinediones, alpha-glucosidase inhibitors, and sodium-glucose cotransporter-2 (SGLT2) inhibitors are the commonly used oral antidiabetic agents (OADs) both as mono- and combination-therapy in T2DM patients.[4] Among all the available OADs, SGLT2 inhibitors are the only ones which target the impaired glucose reabsorption in kidney, an important component of the ominous octet.[5]
Sodium-glucose cotransporter-2 inhibitors and their mechanism of action
Glucose reabsorption by the kidneys is mediated by specific glucose transport proteins, in particular, SGLT2.[5] In individuals with T2DM, the capacity to reabsorb glucose and the plasma glucose concentration at which renal excretion of glucose occurs (i.e., the threshold) are elevated possibly due to upregulation of the expression of SGLT2 in the proximal tubule.[5] Therefore, inhibition of glucose reabsorption and augmentation of renal excretion of glucose are the methods by which this physiological disorder can possibly be corrected.[5] SGLT2 inhibitors are a novel class of drugs which promote renal excretion of glucose and thereby decrease elevated blood glucose levels in patients with T2DM.[5]
Currently, three SGLT2 inhibitors, namely, canagliflozin, dapagliflozin, and empagliflozin, have been approved by the US Food and Drug Administration (FDA) for monotherapy and combination therapy in patients with T2DM.[6] The Research Society for the Study of Diabetes in India (RSSDI) has also recommended the use of SGLT2 inhibitors in the patients with T2DM.[7]
This review explores the recommended guidelines for combination therapy, advantages of combination or fixed-dose combination (FDC) therapy, and the role of SGLT2 inhibitors as a choice of drug as a combination with other OADs. The review further explores the effectiveness and safety of SGLT2 inhibitors when used as dual and triple combination therapy in patients with T2DM and drug–drug interactions between SGLT2 inhibitors and other OADs.
METHODS
Electronic database, PubMed, was searched (from January 2011 to August 2016) to identify relevant studies evaluating the effectiveness and long-term outcomes of SGLT2 inhibitors in combination with other glucose-lowering agents for the treatment of T2DM. Predefined MeSH terms such as SGLT2 inhibitors AND combination therapy AND T2DM AND oral anti-diabetic agents, SGLT2 inhibitors AND metformin, SGLT2 inhibitors AND DPP-4i, SGLT2 inhibitors AND sulphonyl urea, SGLT2 inhibitors AND insulin, SGLT2 inhibitors and GLP-1 inhibitors, and SGLT2 inhibitors AND triple combination were used for an extensive database search. The search was limited to articles published in English language for the last 5 years and to those studies conducted in humans.
Selection criteria
The search using the above-mentioned criteria resulted in 82 articles. The titles and abstracts of the articles were evaluated to identify relevant articles for inclusion in the present review. Fourteen articles (including case reports and articles which studied non-FDA approved SGLT2 inhibitors) were excluded. The remaining 68 articles were selected for further evaluation. A total of 43 studies (including narrative reviews, commentaries, and studies which were already included in other higher level of evidences such as systematic reviews and meta-analysis) were further eliminated. A total of 25 articles (including 19 clinical trials, two meta-analyses, three systematic reviews, and one meta-analysis and systematic review) were evaluated in the current review.
Current treatment guidelines for using combination therapy in type 2 diabetes mellitus
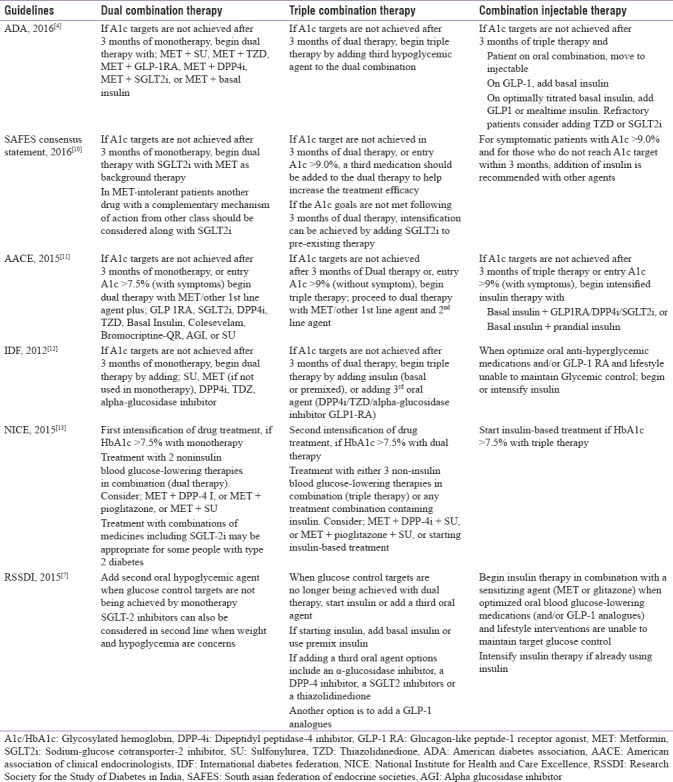
Due to the progressive nature of T2DM and increasing dysfunction of insulin-secreting pancreatic β-cells, most patients fail to achieve optimal glycemic control with monotherapy (oral or injectable), and hence, combination therapy (by adding another OAD) is preferred for the treatment of patients with T2DM.[8] The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend initiation of “dual combination therapy” if HbA1c targets are not achieved after 3 months of monotherapy and proceed to “triple combination therapy” if HbA1c targets are not achieved even after 3 months of dual therapy. Furthermore, it is recommended that insulin (basal or prandial) should be added along with the oral glycemic agent if the target HbA1c levels are not attained after 3 months of triple combination therapy [Table 1].[4,9] Recommendations by the American Association of Clinical Endocrinologists (AACE), SAFES consensus statement, the International Diabetes Federation, the National Institute for Health and Care Excellence, and RSSDI were also found to be similar to the ADA and EASD recommendations for the use of combination therapy in T2DM patients [Table 1].[7,10,11,12,13] In addition, AACE encourages the initiation of dual therapy if HbA1c targets are not achieved after 3 months of monotherapy, or if entry HbA1c >7.5% (with symptoms). Furthermore, AACE recommends initiation of triple combination therapy if HbA1c targets are not achieved after 3 months of dual combination therapy, or if entry HbA1c >9% (without symptom).[11]
Table 1.
Guidelines for management of type 2 diabetes by combination drug therapy
Advantages of combination or fixed-dose combination therapy
Early achievement of glycemic goals, lesser chance of having dose-related side effects (because of a submaximal dose of each agent used), and sustained maintenance of euglycemia are the major advantages associated with early initiation of combination therapy.[14] A systematic review which included data from five randomized controlled trials showed that initiation of combination therapy with SGLT2 inhibitors had a statistically significant impact on the reduction in HbA1c level (weighted mean differences [WMDs] −0.43%, 95% confidence interval [CI] −0.56, −0.30) as compared with monotherapy in patients with T2DM.[15]
Oral combination therapy can be administered as separately dispensed, individual medications or as FDCs (i.e., a single-pill formulation) which are available for many glucose-lowering agents. The major advantages of FDCs are greater efficacy compared to high-dose monotherapies, reduced complexity of dosing regimens and thereby better patient adherence to the treatment, fewer chances of having adverse drug reactions, and cost-effectiveness.[16]
Combination therapy with sodium-glucose cotransporter-2 inhibitors: Efficacy and safety
Due to their unique mechanism of action, the glucose-lowering effect of SGLT2 inhibitors remains independent of β-cell function and insulin sensitivity, thereby reducing chances of severe hypoglycemia in patients receiving these agents.[5] Since SGLT2 inhibitors do not usually cause hypoglycemia in the absence of therapies that otherwise cause hypoglycemia, they become an ideal choice of the drug when used in combination with other glucose-lowering agents.[17,18]
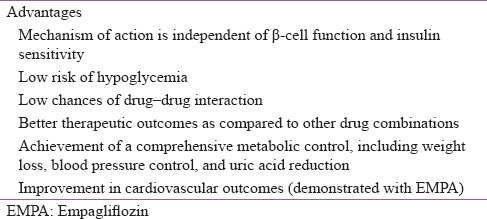
In addition, due to calorie restriction mimicry, and physiologically regulated ketogenesis, SGLT2 inhibitors (particularly empagliflozin) have shown beneficial effects on weight loss, cardiovascular outcomes, and all--cause mortality in patients with T2DM.[19] The combination of SGLT2 inhibitors and other OADs complements each other due to the unique mechanism of action and low risk of hypoglycemia with SGLT2 inhibitors [Table 2].[20]
Table 2.
Advantages of sodium-glucose cotransporter-2 inhibitors when used in combination therapy
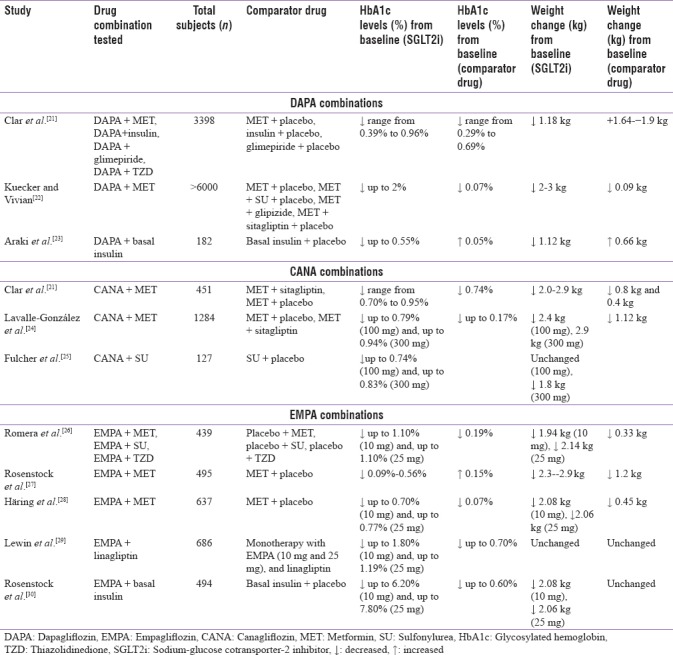
A systematic review of clinical trials evaluating the efficacy of SGLT2 inhibitors as dual or triple therapy in T2DM patients reported better outcomes with combination therapies of SGLT2 inhibitors. Combination therapy with dapagliflozin (10 mg) led to better reduction in HbA1c, weight loss (in conjunction with advice on lifestyle and diet), systolic blood pressure, and fasting plasma glucose (FPG) levels compared to the combination therapy with placebo [Table 3].[21] Also, combination therapy with canagliflozin reduced HbA1c levels to a greater extent as compared to combination therapy with sitagliptin and led to better weight reduction [Table 3].[21] Another study which evaluated the efficacy of empagliflozin as combination therapy in T2DM patients reported a better efficacy and safety profile when used as a combination therapy (as an add-on or single-pill therapy) with other OADs (DPP-4i and metformin). Across all the trials included in this study, empagliflozin combination therapies were well tolerated and had a low rate of hypoglycemia in the patients.[31] Pooled analysis of three safety studies which included elderly (>65 years) and overweight/obese patients with uncontrolled T2DM showed that empagliflozin when combined with other OADs (metformin, SU, and pioglitazone) significantly reduced HbA1c and body weight and was well tolerated [Table 3].[26]
Table 3.
Dual combination therapy with sodium-glucose cotransporter-2 inhibitors
A comparative systematic review and meta-analysis of 14 studies (n = 6980) was conducted to compare the efficacy and safety of SGLT2 inhibitors and DPP4i as a combination with other OADs. Results of this study showed greater reduction in HbA1c (WMD −0.24%, 95% CI −0.43–−0.05%), FPG (WMD −18.0 mg/dL, 95% CI −28.5–−7.6 mg/dL) and body weight (WMD −2.38 kg, 95% CI −3.18–−1.58 kg) from baseline with SGLT2 inhibitor than with DPP4i without increasing the risk of hypoglycemia (relative risks 1.19, 95% CI 0.78–1.82).[32]
Safety studies have also shown that combination therapies of SGLT2 inhibitors have been well tolerated and did not result in any severe adverse events (AEs) (e.g., renal impairment, fractures, malignant neoplasm, and volume-related events).[31,33] Furthermore, the frequency of AEs remained almost similar for monotherapy (79.1% with dapagliflozin) and combination therapy (72.4% with dapagliflozin plus other hypoglycemic agents).[33]
For a better understanding of the strengths and limitations of SGLT2 inhibitors in combination therapy, the subsequent sections elaborate the different dual and triple combinations of SGLT2 inhibitors with other hypoglycemic agents.
Dual combination therapy with sodium-glucose cotransporter-2 inhibitors
Sodium-glucose cotransporter-2 inhibitors and metformin
Metformin is a biguanide that suppresses hepatic glucose production (HGP) via inhibition of gluconeogenesis. It increases insulin sensitivity, peripheral glucose uptake, and decreases the absorption of glucose from the gastrointestinal tract resulting in decreased fasting glucose levels and HbA1c levels.[3] The mechanism of action of metformin is different from SGLT2 inhibitors; both drugs complement each other's action and neither of these compounds target pancreatic β-cells, increase body weight, or cause major safety risks.[3] A systematic review of 14 studies reported that the combination of SGLT2 inhibitor (dapagliflozin) and metformin resulted in a decrease in HbA1c levels, weight loss, and modest systolic blood pressure decrease of 3–5 mmHg in patients with T2DM [Table 3].[22]
A study comparing the effectiveness of canagliflozin as an add-on to metformin with placebo and sitagliptin demonstrated that canagliflozin at the doses of 100 mg and 300 mg was noninferior to sitagliptin. In addition, it was reported that canagliflozin was superior to sitagliptin after 52 weeks [Table 3].[24] Another study evaluating empagliflozin as an add-on to stable background metformin therapy reported that the empagliflozin was superior to placebo in reducing HbA1c levels and inducing weight loss in patients with T2DM [Table 3].[28]
Sodium-glucose cotransporter-2 inhibitors and sulfonylureas
Sulphonylureas (SU) cause glucose independent closure of the adenosine triphosphate-sensitive K-channels and release of insulin by binding to the sulfonylurea receptor 1 on the pancreatic β-cells. With modern SU, the risk of weight gain and hypoglycemia are minimnal as long as the drugs are used in safe and smart manner.[34] Proper patient selection, correct choice of drug and dose, patient education and empowerment, and physician training are the few points that ensures effective and safe use of SU as monotherapy and in combination with other OADs.[34]
In a placebo-controlled, double-blinded trial with SGLT2 inhibitor (canagliflozin) added to ongoing SU monotherapy, it was reported that this combination improved HbA1c levels and led to better reduction in body weight [Table 3].[25] The episodes of hypoglycemia were more with placebo compared to canagliflozin. Out of total 127 subjects, 15% with canagliflozin 300 mg, 0% with canagliflozin 100 mg, and 4.4% with placebo reported hypoglycemia. However, mild to moderate AEs like male and female genital mycotic infections, pollakiuria, and thirst were more common with canagliflozin combination therapy.[25]
Sodium-glucose cotransporter-2 inhibitors and dipeptidyl peptidase-4 inhibitors
The DPP-4i constrain the enzyme that degrades incretin hormones, glucagon-like peptide-1 receptor agonists (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), thereby resulting in elevated plasma GLP-1 and GIP concentrations and leading to inhibition of endogenous glucose production and reduction in plasma glucose concentration.[35]
The DPP-4i and SGLT2 inhibitors have complementary mechanism of actions, and hence this combination can be used to achieve better glycemic control in T2DM patients. When SGLT2 inhibitor is added to an ongoing DPP-4i therapy, it provides better glucose control with low risk of AEs like hypoglycemia, weight gain, and risk of heart failure. On the other hand, for patients with ongoing SGLT2 inhibitors therapy, addition of a DPP-4i may have a potential to reduce endogenous glucose production and enhance glucose-lowering ability of SGLT2 inhibitors.[36]
A randomized trial was conducted to evaluate the efficacy of dapagliflozin (10 mg) alone, saxagliptin (5 mg) alone, or a combination of saxagliptin and dapagliflozin in poorly controlled, metformin-treated T2DM patients (n = 534) for 24 weeks.[37] Results of this study demonstrated that combination of SGLT2 inhibitor and DPP-4i has the potential to reduce HbA1c levels to a significant extent [Table 3].[37] In addition, dapagliflozin alone or in combination with saxagliptin demonstrated a modest weight loss and a small decrease in systolic blood pressure (2–4 mmHg), while saxagliptin alone had no effect on blood pressure or body weight. However, no significant difference was observed in the FPG and postprandial plasma glucose concentrations with monotherapy and combination therapy.[37]
Similarly, a single pill combination of empagliflozin and linagliptin was studied in treatment-naïve patients with T2DM. The reduction in HbA1c level was greater with the combination of empagliflozin 25 mg and linagliptin 5 mg as compared with linagliptin 5 mg alone [Table 3].[37] In addition, better reduction in HbA1c levels was observed for empagliflozin 10 mg and linagliptin 5 mg combination as compared with the individual components (i.e., empagliflozin and linagliptin alone) [Table 3].[29] The combination of linagliptin plus empagliflozin improves five pathophysiological disturbances in T2DM patients, namely, increased incretin effect, enhanced insulin secretion, decreased glucagon secretion, suppression of the elevated rate of HGP, and reduction of the elevated threshold for glucose spillage into the urine, leading to decreased reabsorption of glucose by the diabetic kidney.[29]
Sodium-glucose cotransporter-2 inhibitors and GLP-1 receptor agonists
The GLP-1 agonists decrease β-cell workload and decrease the demand for insulin secretion by slowing down gastric emptying, reducing peak nutrient absorption, and insulin demand.[38] The combination of SGLT2 inhibitor and GLP-1 receptor agonists is anticipated to be ideal for inducing weight loss in patient with T2DM. However, till date, no major trial is published which has studied this combination. Few reports published so far indicated that clinicians are trying the combination of SGLT2 inhibitors and GLP-1 in clinical practice, and initial reports suggested that additional weight loss is typically observed. However, the sample size of these studies is too low to offer robust conclusions regarding the safety or utility of this combination.[39] Since GLP1 receptor agonist reduce glucagon and SGLT2 inhibitors increase glucagon, co-therapy with both may alter this concern. Hence, the ADA and ESDA guidelines (2016), does not recommend combination therapy with GLP1 receptor agonist and SGLT2 inhibitors in T2DM patients.[4,9]
Sodium-glucose cotransporter-2 inhibitors and basal insulin
Insulin therapy has remained a mainstay for the treatment of T2DM in patients with insufficient glycemic control with OADs. The SGLT2 inhibitor (dapagliflozin) when used as add-on treatment along with insulin therapy showed significantly greater reduction in HbA1c levels, FPG levels, and body weight without severe hypoglycemia compared with the placebo at week 16 [Table 3].[23] In this study, the baseline insulin dose remained fixed, unless there were safety concerns. It was observed that the use of dapagliflozin in addition to insulin reduced the total insulin dose to a considerable extent in the subjects. The reduction in total insulin dose may be due to the hypoglycemic effect of dapagliflozin. It is important to mention that dose reduction of insulin is only possible if insulin dose titration is allowed in such patients. In addition, the study results showed that the dose of insulin decreased in the dapagliflozin group and increased in the placebo group over a period of 16 weeks.[23]
Another study which evaluated empagliflozin 10 mg and 25 mg in combination with basal insulin showed significant reductions in HbA1c and FPG, and improvement in body weight and systolic blood pressure (P = 0.004) as compared with placebo [Table 3].[30] During this study, minimal insulin adjustments were made because there was no glucose monitoring committee enforcing insulin titrations. This reflects the real world clinical practice, in which insulin algorithms are not systematically executed.[30]
Triple combination therapy with sodium-glucose cotransporter-2 inhibitors
Metformin, sodium-glucose cotransporter-2 inhibitors and sulphonylurea
The combination of metformin, SGLT2 inhibitors, and SU is a popular choice of treatment in patients with T2DM. The SGLT2 inhibitor (empagliflozin), when used as an add-on to SUs and metformin in a 24-week study, showed improvement in glucose control, reduction in body weight, and decrease in systolic blood pressure as compared with placebo [Table 4].[27]
Table 4.
Triple combination therapy with sodium-glucose cotransporter-2 inhibitors
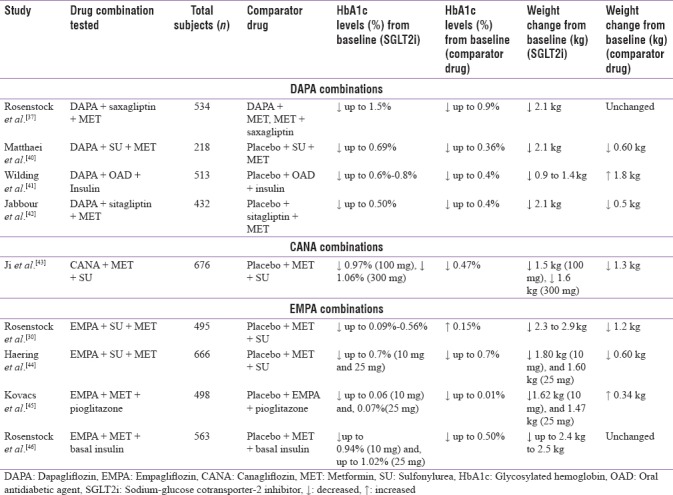
Another study showed that dapagliflozin was well tolerated and effective over 24 weeks as an add-on to metformin and SU. A higher proportion of patients achieved therapeutic glycemic response as compared with placebo [Table 4]. Body weight and systolic blood pressure also showed a reduction from baseline over 8 and 24 weeks, with dapagliflozin [Table 4].[40]
Metformin, sodium-glucose cotransporter-2 inhibitors, and thiazolidinedione
A randomized, placebo-controlled study of empagliflozin as an add-on to pioglitazone or pioglitazone and metformin in patients with T2DM showed that the triple combination can result in significant reductions in HbA1c levels, weight, and blood pressure, with a low risk of hypoglycemia. However, due to the addition of SGLT2 inhibitor, the incidence of genital mycotic infection remains high in this population [Table 4].[45]
Metformin, sodium-glucose cotransporter-2 inhibitors, and DPP-4 inhibitors
A 24-week, multicenter, randomized, double-blinded trial conducted to assess the efficacy and safety of dapagliflozin as an add-on therapy in T2DM patients with inadequately controlled glycemic levels while on DPP-4 inhibitor (with or without metformin) reported that dapagliflozin significantly reduced mean HbA1c levels and body weight as compared with placebo [Table 4].[42]
Metformin, sodium-glucose cotransporter-2 inhibitors, and basal insulin
A 52-week study of T2DM patients with inadequately controlled plasma glucose levels despite being administered multiple daily injections of insulin (with or without metformin) reported that the add-on therapy with empagliflozin 10 mg and 25 mg improved the glucose control and reduced body weight as compared with placebo and insulin alone [Table 4]. Majority of the patients attained HbA1c <7% with empagliflozin [Table 4]. In addition, empagliflozin 10 mg and empagliflozin 25 mg reduced insulin doses (−9–−11 IU/day) and weight (−2.4 to −2.5 kg) versus placebo (all P < 0.01) at week 52.[46]
Drug–Drug interactions between sodium-glucose cotransporter-2 inhibitors and other hypoglycemic agents
Pharmacokinetic studies have shown that SGLT2 inhibitors can be combined with any other glucose-lowering agent.[47] Dapagliflozin can be combined with pioglitazone, metformin, glimepiride, or sitagliptin without dose adjustment of either drug. It was found that other hypoglycemic agents do not impact pharmacokinetics of dapagliflozin (only a slight [+8%] greater exposure to dapagliflozin after co-administration of sitagliptin was observed without any clinical relevance).[47] Similarly, dapagliflozin did not influence the pharmacokinetic parameters of the other glucose-lowering agents (only a trend for a slightly greater exposure to glimepiride was detected).[47] The addition of dapagliflozin in patients who were insufficiently controlled with glimepiride resulted in reduction of HbA1c levels with only a slight increase in the risk of hypoglycemia. Likewise, another study demonstrated that α-glucosidase inhibitor did not modify the pharmacokinetics of dapagliflozin.[48]
A few drug interactions have been reported with canagliflozin. It was reported that metformin slightly increased exposure to canagliflozin (peak plasma concentrations +5%; area under the concentration-time curve +10%). On the other hand, canagliflozin was not found to modify the exposure to metformin. The changes reported were modest without any clinical relevance.[49]
In pharmacokinetics studies, empagliflozin has been tested in single-dose when co-administered with various other glucose-lowering agents (such as metformin, glimepiride, sitagliptin, and linagliptin).[50] It was reported that glimepiride slightly decreased the exposure of empagliflozin, whereas sitagliptin modestly increased it.[27,51] However, these changes were too small for being considered as clinically relevant. In addition, it was found that empagliflozin did not modify exposure to other glucose-lowering agents tested.[50,51]
CONCLUSION
The challenging pathogenesis of T2DM and limitations of the available OADs have emphasized the need for better therapeutic options and strategies for adequate management of this condition. Involvement of multiple metabolic pathways in pathogenesis has restricted the therapeutic efficacy of any hypoglycemic drug when used as monotherapy. Hence, a combination of two or more agents with different mechanisms of action should be considered to achieve optimal plasma glucose levels in patients with T2DM.
SGLT2 inhibitors are one such novel class of drugs which have shown valuable efficacy profile when used as an add-on with other OADs. Multiple trials have demonstrated that SGLT2 inhibitors can be flexibly combined with other agents to achieve better HbA1c levels, weight loss, and blood pressure control. The comparatively better safety profile of SGLT2 inhibitors and their unique mechanism of action have made them a good therapeutic option for T2DM patients who fail to achieve adequate glycemic control with monotherapies. However, the safety of SGLT2 inhibitors in combination with other agents is yet to be established. Larger clinical trials and real-world evidence solutions such as observational studies can help attain data and add more to the available literature.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge AstraZeneca Pharma India Ltd., and Turacoz, Healthcare Solutions for Medical writing and editing support.
REFERENCES
- 1.WHO Diabetes Fact Sheet (Revised June 2016) [Last accessed on 2016 Jul 10]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/
- 2.Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36(Suppl 2):S127–38. doi: 10.2337/dcS13-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association (ADA) Diabetes Guidelines. 2016. [Last accessed on 2016 Jul 10]. Available from: http://www.ndei.org/uploadedFiles/Common/NDEI/Treatment_Guidelines/ADA%202015%20Summary%20PDF.pdf .
- 5.Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: A growing class of antidiabetic agents. Drugs Context. 2014;3:212264. doi: 10.7573/dic.212264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. Drug Safety and Availability. Post-market Drug Safety Information for Patients and Providers. Sodium-glucose Cotransporter-2 (SGLT2) Inhibitors. [Last accessed on 2016 Aug 10]. Available from: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm446852.htm .
- 7.Madhu SV, Saboo B, Makkar BM, Reddy GC, Jana J, Panda JK. RSSDI Clinical Practice recommendations for management of type 2 diabetes mellitus, 2015. Guidelines. [Last accessed on 2016 Aug 10];Int J Diabetes Dev Ctries. 2015 35(Suppl 1):1–71. Available from: http://www.link.springer.com/article/10.1007/s13410-015-0450-9 . [Google Scholar]
- 8.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.adults? Diabetes Care. 2008;31:81–6. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 9.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 10.Kalra S, Ghosh S, Aamir AH, Ahmed MT, Amin MF, Bajaj S, et al. Safe and pragmatic use of sodium-glucose co-transporter 2 inhibitors in type 2 diabetes mellitus: South Asian Federation of Endocrine Societies consensus statement. Indian J Endocrinol Metab. 2017;21:210–230. doi: 10.4103/2230-8210.196029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology On The Comprehensive Type 2 Diabetes Management Algorithm-2016 executive summary. Endocr Pract. 2016;22:84–113. doi: 10.4158/EP151126.CS. [DOI] [PubMed] [Google Scholar]
- 12.International Diabetes Federation, Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. 2012. [Last accessed on 2016 Aug 10]. Available from: http://www.idf.org/sites/default/files/IDF-Guideline-for-Type-2-Diabetes.pdf .
- 13.National Institute of Health Care and Excellence (NICE) Guidelines for Type 2 Diabetes in Adults: Management (NG-28) [Last accessed on 2016 Aug 10]. Available from: https://www.nice.org.uk/guidance/ng28 .
- 14.Lavernia F, Adkins SE, Shubrook JH. Use of oral combination therapy for type 2 diabetes in primary care: Meeting individualized patient goals. Postgrad Med. 2015;127:808–17. doi: 10.1080/00325481.2015.1085293. [DOI] [PubMed] [Google Scholar]
- 15.Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: Systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:410–7. doi: 10.1111/dom.12233. [DOI] [PubMed] [Google Scholar]
- 16.Bell DS. Combine and conquer: Advantages and disadvantages of fixed-dose combination therapy. Diabetes Obes Metab. 2013;15:291–300. doi: 10.1111/dom.12015. [DOI] [PubMed] [Google Scholar]
- 17.Davidson JA, Kuritzky L. Sodium glucose co-transporter 2 inhibitors and their mechanism for improving glycemia in patients with type 2 diabetes. Postgrad Med. 2014;126:33–48. doi: 10.3810/pgm.2014.10.2819. [DOI] [PubMed] [Google Scholar]
- 18.Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85:520–6. doi: 10.1038/clpt.2008.251. [DOI] [PubMed] [Google Scholar]
- 19.Kalra S, Jain A, Ved J, Unnikrishnan AG. Sodium-glucose cotransporter 2 inhibition and health benefits: The Robin Hood effect. Indian J Endocrinol Metab. 2016;20:725–729. doi: 10.4103/2230-8210.183826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheen AJ. Drug-drug interactions with sodium-glucose cotransporters type 2 (SGLT2) inhibitors, new oral glucose-lowering agents for the management of type 2 diabetes mellitus. Clin Pharmacokinet. 2014;53:295–304. doi: 10.1007/s40262-013-0128-8. [DOI] [PubMed] [Google Scholar]
- 21.Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001007. pii: e001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuecker CM, Vivian EM. Patient considerations in type 2 diabetes – Role of combination dapagliflozin-metformin XR. Diabetes Metab Syndr Obes. 2016;9:25–35. doi: 10.2147/DMSO.S81565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki E, Onishi Y, Asano M, Kim H, Ekholm E, Johnsson E, et al. Efficacy and safety of dapagliflozin in addition to insulin therapy in Japanese patients with type 2 diabetes: Results of the interim analysis of 16-week double-blind treatment period. J Diabetes Investig. 2016;7:555–64. doi: 10.1111/jdi.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia. 2013;56:2582–92. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fulcher G, Matthews DR, Perkovic V, de Zeeuw D, Mahaffey KW, Weiss R, et al. Efficacy and safety of canagliflozin used in conjunction with sulfonylurea in patients with type 2 diabetes mellitus: A randomized, controlled trial. Diabetes Ther. 2015;6:289–302. doi: 10.1007/s13300-015-0117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romera I, Gomis R, Crowe S, de Pablos-Velasco P, Aranda U, García A, et al. Empagliflozin in combination with oral agents in young and overweight/obese type 2 diabetes mellitus patients: A pooled analysis of three randomized trials. J Diabetes Complications. 2016;30:1571–6. doi: 10.1016/j.jdiacomp.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154–60. doi: 10.1111/dom.12185. [DOI] [PubMed] [Google Scholar]
- 28.Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–9. doi: 10.2337/dc13-2105. [DOI] [PubMed] [Google Scholar]
- 29.Lewin A, DeFronzo RA, Patel S, Liu D, Kaste R, Woerle HJ, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care. 2015;38:394–402. doi: 10.2337/dc14-2365. [DOI] [PubMed] [Google Scholar]
- 30.Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. EMPA-REG BASALTM trial investigators. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: A 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–48. doi: 10.1111/dom.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hershon KS. Options for empagliflozin in combination therapy in type 2 diabetes mellitus. Int J Gen Med. 2016;9:155–72. doi: 10.2147/IJGM.S100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min SH, Yoon JH, Hahn S, Cho YM. Comparison between SGLT2 inhibitors and DPP4 inhibitors added to insulin therapy in type 2 diabetes: A systematic review with indirect comparison meta-analysis. Diabetes Metab Res Rev. 2017;33:1–11. doi: 10.1002/dmrr.2818. [DOI] [PubMed] [Google Scholar]
- 33.Kaku K, Maegawa H, Tanizawa Y, Kiyosue A, Ide Y, Tokudome T, et al. Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: An open-label study. Diabetes Ther. 2014;5:415–33. doi: 10.1007/s13300-014-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalra S, Aamir AH, Raza A, Das AK, Azad Khan AK, Shrestha D, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: A consensus statement. Indian J Endocrinol Metab. 2015;19:577–96. doi: 10.4103/2230-8210.163171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balas B, Baig MR, Watson C, Dunning BE, Ligueros-Saylan M, Wang Y, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92:1249–55. doi: 10.1210/jc.2006-1882. [DOI] [PubMed] [Google Scholar]
- 36.Scheen AJ. DPP-4 inhibitor plus SGLT-2 inhibitor as combination therapy for type 2 diabetes: From rationale to clinical aspects. Expert Opin Drug Metab Toxicol. 2016;12:1407–17. doi: 10.1080/17425255.2016.1215427. [DOI] [PubMed] [Google Scholar]
- 37.Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: A randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376–83. doi: 10.2337/dc14-1142. [DOI] [PubMed] [Google Scholar]
- 38.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 39.Mcgovern AP, Dutta N, Watters K, Munro N, Feher M. Additive weight loss effect with a combination of an oral sodium-glucose cotransporter 2 (SGLT2) inhibitor and a glucagon-like peptide 1 (GLP-1) agonist in Type 2 diabetes. Diabet Med. 2015;32:2–3. [Google Scholar]
- 40.Matthaei S, Bowering K, Rohwedder K, Grohl A, Parikh S Study Group. Dapagliflozin improves glycemic control and reduces body weight as add-on therapy to metformin plus sulfonylurea: A 24-week randomized, double-blind clinical trial. Diabetes Care. 2015;38:365–72. doi: 10.2337/dc14-0666. [DOI] [PubMed] [Google Scholar]
- 41.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin Study Group Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: Efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–36. doi: 10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 42.Jabbour SA, Hardy E, Sugg J, Parikh S Study Group. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: A 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37:740–50. doi: 10.2337/dc13-0467. [DOI] [PubMed] [Google Scholar]
- 43.Ji L, Han P, Liu Y, Yang G, Dieu Van NK, Vijapurkar U, et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17:23–31. doi: 10.1111/dom.12385. [DOI] [PubMed] [Google Scholar]
- 44.Haering HU, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, et al. Empagliflozin as add-on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;110:82–90. doi: 10.1016/j.diabres.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 45.Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: A 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–58. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 46.Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–23. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 47.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: A randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928–38. doi: 10.1111/j.1463-1326.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- 48.Imamura A, Kusunoki M, Ueda S, Hayashi N, Imai Y. Impact of voglibose on the pharmacokinetics of dapagliflozin in Japanese patients with type 2 diabetes. Diabetes Ther. 2013;4:41–9. doi: 10.1007/s13300-012-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devineni D, Sarich TC, Wexler D, Shalayda K, Ghosh A, Skee D, et al. Presented at the American Diabetes Association (ADA) 71st Scientific Sessions. San Diego, CA: 2011. Jun 24-28, Effects of Canagliflozin on the Pharmacokinetics (PK) and Pharmacodynamics (PD) of Metformin and Glyburide [Abstract No. 2268-PO 2011] [Google Scholar]
- 50.Brand T, Macha S, Mattheus M, Pinetti S, Woerle HJ. Pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 inhibitor, and glimepiride following co-administration in healthy volunteers: A randomised, open-label, crossover study. Diabetes Res Clin Metab. 2012;1:1–7. [Google Scholar]
- 51.Brand T, Macha S, Mattheus M, Pinnetti S, Woerle HJ. Pharmacokinetics of empagliflozin, a sodium glucose cotransporter-2 (SGLT-2) inhibitor, coadministered with sitagliptin in healthy volunteers. Adv Ther. 2012;29:889–99. doi: 10.1007/s12325-012-0055-3. [DOI] [PubMed] [Google Scholar]


