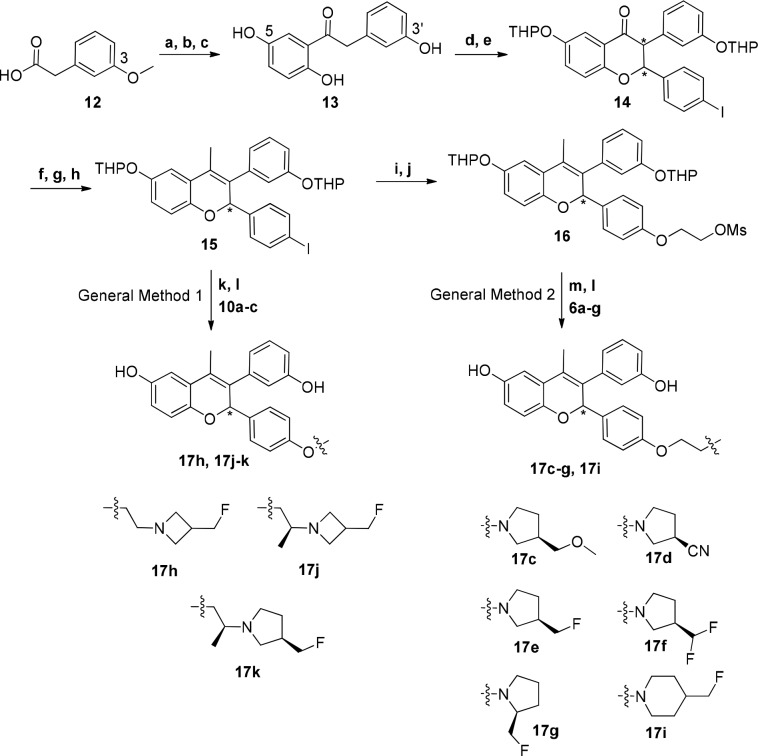
Scheme 1. Preparation of Chromene ER Ligands.
Reagents and conditions: (a) SOCl2, DMF, DCM, 0 °C; (b) 1,4-dimethoxybenzene, AlCl3, DCM, 0 °C, 55%; (c) BBr3, DCM, −78 to 0 °C, 57%; (d) DHP, PPTS, DCM, 70%; (e) 4-iodobenzaldehyde, piperidine, DBU, s-butanol, reflux, 75%; (f) MeMgCl, THF, 0 °C to rt, 98%; (g) 80% AcOH/water, 90 °C, 67%; (h) DHP, PPTS, DCM, 55%; (i) ethane-1,2-diol, CuI, 1,10-phenantroline, K2CO3, butyronitrile, 125 °C, 58%; (j) methanesulfonyl chloride, TEA, DCM, 0 °C, 88%; (k) 17h, 17j–k: CuI, K2CO3, butyronitrile, 125–135 °C, 2–4 d, 75–82%; (l) 17c–g, 17i: K2CO3, acetonitrile, 60 °C to reflux, 62–76%; (m) 80% AcOH/water, rt, 27–85%.