Abstract
Background:
The public health burden of Enterotoxigenic Escherichia coli (ETEC) is high but no vaccine is specifically approved to prevent ETEC infections.
Methods:
We performed a Phase 1, dose escalation study (1 to 50 μg) evaluating the sublingual (SL) delivery of the double mutant heat-labile toxin LTR192G/L211A (dmLT) in 80 healthy adult volunteers. The primary objective was safety and the secondary was the immunogenicity of the dmLT. Subjects received 3 doses of dmLT at days 1, 15, and 29. Subjects receiving the first dose at each dosage level were observed overnight in a research facility. The second and third doses were administered on an outpatient basis. Data from cohorts 1–4 were used to select the cohort 5 dose (25 μg), comparing SL and oral routes.
Results:
The vaccine appeared safe and well tolerated with only rare development of vomiting or diarrhea. The serum anti-dmLT IgA and IgG and neutralizing antibody responses were modest after any of the SL immunizations. Serum IgA and IgG titers were increased at the higher antigen doses (25 or 50 μg) but the percent with 4-fold increases was at best 38% for both IgA and IgG.. The 4-fold increase among subjects receiving all 3 doses was 43% for both IgA and IgG.. Antibody titers following oral administration were, in general, significantly higher than after SL. The frequency of IgA- or IgG-ASCs in circulation were somewhat vaccine dose dependent and were detected at a moderate level. However, antibodies in saliva or stool were rarely detected. Post-vaccination increases in T cells or cytokine production were also infrequent.
Conclusion:
The dmLT vaccine formulation evaluated here was safe but only moderately immunogenic at doses up to 50 μg when administered by the SL or oral route. Studies at higher doses with better formulations appear warranted.
Keywords: ETEC, Escherichia coli, vaccine, sublingual, oral, dmLT
Introduction
Despite the public health burden of Enterotoxigenic Escherichia coli (ETEC) on travelers, deployed soldiers and, most significantly, young children in low-resource settings, there is no vaccine specifically licensed to prevent ETEC disease. One of the principal ETEC virulence factors, heat-labile enterotoxin (LT), has been studied as a potential vaccine antigen [1]. Subtoxic doses or attenuated forms of this protein have also been shown to retain adjuvant activity in pre-clinical animal studies and human trials [2–5].
LT is a multimeric (1 A-subunit: 5 B-subunits) enzyme that is similar to cholera toxin. To modify the toxin for potential use as a vaccine, a single mutant LT (mLT) was created to disrupt the enzymatic and toxigenic activity of LT [2, 6]. In preclinical studies, the single mutant LTR192G demonstrated reduced toxicity and retained its adjuvant properties [3]. Early clinical trials, however, were associated with mild, self-limited diarrhea [7, 8]. Therefore, an additional mutation was introduced in a putative pepsin-sensitive proteolytic site in the A2 domain [4]. This double mutant, LTR192G/L211A, or dmLT, demonstrated adjuvanticity in mice at levels comparable to mLT in an oral H. pylori vaccine study [9]. LT has been shown to be immunogenic in animals and limited human trials [10, 11] and protective in animal models [12], and is relatively easy to produce. In addition, recent field studies of cholera vaccines which induce cross-reactive anti-LT toxin immunity indicate that an anti-LT based vaccine can be protective against a broader array of ETEC pathotypes than originally anticipated [12–14]. Thus, this protein has the potential to be both a stand-alone vaccine as well as a mucosal adjuvant for other co-administered vaccine antigens [5, 15–20] and can safely be given at oral doses up to 100 μg [11].
Recent animal studies have demonstrated that the sublingual (SL) route of immunization induces serum and local intestinal antibodies to vaccine antigens that are comparable or better than those induced by immunization by the intradermal (ID) and oral routes [20, 21]. In addition, compared to oral immunization, sublingual immunization would allow for increased dose-sparing, negate the need for buffering to neutralize gastric acidity, and reduce the cost of the final product. Bypassing the gut by sublingual delivery might also avoid the consequences of enteric enteropathy so prevalent among infants and young children in low-resource settings that serves as a major barrier to effective immunization with oral vaccines. While oral or SL delivery has not been extensively evaluated with native LT preparations because of potential safety issues, the attenuated dmLT mutant is likely to make SL or oral delivery feasible. A single oral dmLT dose of up to 100 μg has already been shown to be very well tolerated in human subjects [11]. In this clinical trial we explored the safety and immunogenicity profile of ascending doses of dmLT given by the SL route and compare these with those induced by a similar dose of dmLT given orally.
Methods
Subjects
Healthy adults, as assessed by history, physical exam, and safety laboratory testing between the ages of 18–45 were recruited at a single site, Cincinnati Children’s Hospital Medical Center. Subjects were tested for Hepatitis B and C and could not have received any prior vaccinations or challenges with E. coli or cholera, or antibiotics within 2 weeks of vaccination. Subjects were also screened for opiates. The complete eligibility criteria are published at https://clinicaltrials.gov/show/NCT02052934.
Vaccine
LTR192G/L211A, or dmLT was produced to cGMP specifications by the Walter Reed Army Institute of Research (WRAIR Pilot BioProduction Facility Forest Glen, MD) and is a derivative of wild-type ETEC heat-labile enterotoxin that was genetically modified by replacing the arginine at amino acid position 192 with glycine and the leucine at amino acid position 211 with alanine. These two amino acid substitutions are in proteolytic cleavage sites which are critical for activation of the secreted toxin molecules. The protein was designated LTR192G/L211. Lot release testing of LTR192G/L211 Lot No. 1575, including assays of protein purity and sterility were performed by WRAIR while biological activity, antigenicity and adjuvanticity was conducted at Tulane University School of Medicine in New Orleans, LA by Dr. John Clements.
Study Design
This was a Phase 1 dose escalation study in healthy adults to determine the safety and immunogenicity of dmLT as a potential ETEC candidate vaccine, administered by the SL route. cohorts 1 and 2 were enrolled simultaneously at a ratio of 1:1 and received 1 μg or 5 μg of the vaccine, respectively, while cohorts 3 and 4 received 25 μg or 50 μg of the vaccine, respectively. Dose escalation followed review of data through day 104, 75 days after the third vaccination. Data from cohorts 1–4 were then used to select the dose for cohort 5, which compared the oral to the SL route. A dose of 25 μg was chosen, and subjects were randomized 1:1.
Subjects in each cohort were kept overnight in a research facility for observation to ensure safety and tolerability through the first 24 hours following the first dose of vaccine. The second (day 15) and third (day 29) doses were administered on an outpatient basis. Safety was assessed by soliciting symptoms using a subject memory aid through day 8 after each dose and for facial nerve disturbance through day 104.
To prepare the dmLT vaccine, the reconstituted vaccine solution (1 mg/mL) was kept on wet ice until diluted in 0.9% normal saline to the appropriate dosing level (SL preparation) or for subsequent administration to subjects in sodium bicarbonate buffer (oral preparation). The diluted product was administered within 4 hours of preparation. Subjects receiving an SL dose fasted for 30 minutes prior to and after dosing, rinsed their mouth with tap water for approximately 10 minutes immediately before vaccine delivery, and then placed a gauze under the tongue for 1 minute. The vaccine was deposited underneath the tongue using a calibrated tuberculin or insulin syringe. After delivery of vaccine, subjects tilted their head forward, chin to chest, for 1 minute. Subjects avoided drinking liquid or rinsing their mouth for 30 minutes following vaccination.
Subjects in cohort 5 who received oral dmLT, fasted for 90 minutes before product administration and then ingested 120 mL of the bicarbonate solution 1 to 5 minutes before vaccination. dmLT was then administered in 30 mL of bicarbonate buffer solution. The subject was not allowed any food or drink for 90 minutes following oral dosing.
Laboratory
Serum, Fecal and Salivary antibodies
Serum dmLT-specific IgA and IgG were measured by ELISA as previously described [11, 22]. Briefly, Immulon II plates were coated with 1 μg/mL of dmLT, washed with PBS-Tween 0.05% (PBST) and blocked with PBS containing 10% non-fat dry milk overnight at 4°C. Samples diluted in PBST 10% non-fat dry milk were added to the plates in duplicate and incubated for 1 h at 37°C. Horseradish Peroxidase (HRP)-labeled goat anti-human IgA or IgG (Jackson ImmunoResearch, West Grove, PA) were used as secondary antibodies and TMB Microwell Peroxidase Substrate (Sera Care, Milford, MA) as substrate. The reaction was stopped with 1M phosphoric acid and absorbance values at 450 nm measured. End-point titers were calculated based on linear regression analysis as the inverse of the dilution corresponding to an absorbance value of 0.2 above the mean of the blanks. Seroconversion was defined as a ≥4-fold increase in antibody titers over baseline.
Total and dmLT-specific IgA were measured in stool supernatants and in saliva as p reviously described [11, 22]. For total IgA determinations, Immulon II plates were coated with goat anti-human IgA from Jackson ImmunoResearch, at 1 μg/ml in PBS. dmLT specific IgA was measured as described above. IgA concentrations were calculated by interpolation of the regression-corrected absorbance values produced by a standard curve from purified human IgA (Calbiochem, Madison, WI). To normalize the results, the ratio of dmLT-specific IgA to total IgA was calculated, and a positive response defined as a ≥4-fold increase of the ratio over the baseline ratio.
Antibodies in lymphocyte supernatants (ALS), Antibody secreting cells (ASC), and ASC homing potential
For ALS measurements, PBMC (1×107 cells/ml in complete RPMI) were incubated for 72h at 37°C and 5% CO2. The supernatants were then collected and stored at −20°C until tested by ELISA as described above. An increase of ≥2-fold over baseline was considered a positive response.
IgA and IgG dmLT-specific ASC were measured by ELISpot using fresh PBMC as previously described [11, 22]. Briefly, PBMC (2.5×105/well) were seeded in MultiScreen-HA plates (Millipore Sigma, Burlington, MA) coated with 1 μg/mL of dmLT and blocked with complete RPMI 1640 (Thermo Fisher Scientific, Waltham, MA). Plates were then washed with PBS, and cells added to quadruplicate wells. After overnight incubation, plates were washed and HRP- labeled goat anti-human IgA or IgG (Jackson ImmunoResearch) was added for 1h at 37°C. After washing, True Blue Substrate (Sera Care) was added for 5 min, removed, and plates washed and allowed to air dry. Cells seeded in uncoated wells and on wells coated with goat anti-human IgA and IgG were included as controls. Results were reported as the number of IgA or IgG spot forming cells (SFC) per 106 PBMC. A positive ASC response was defined as >8 SFC per 106 cells. To determine dmLT-specific ASC with homing capacity, B cells expressing gut homing molecule integrin α4β7 were sorted from total PBMC, as previously described [23], and ASC measured as described above.
LT-Toxin Neutralization
LT-toxin neutralizing antibodies were measured using an assay adapted from Glenn et al. [24]. Briefly, Y-1 Adrenal Cells (ATCC, Manassas, VA) were grown in Ham’s F-12k media supplemented with 15% horse serum and 2.5% fetal bovine serum (Thermo Fisher Scientific) and harvested using 0.25% trypsin (Sigma). Serum samples were serially diluted 2-fold (starting at a 1:2) in Ham’s F-12k media and incubated with 5 ng/ml LT (Berna Biotech, Berne, Switzerland) for 30 min at 37°C. Y-1 cells (2.5×104 cells) were added to each well and plates were incubated for 15–18 h at 37°C. Cells susceptible to LT become rounded. End-point titers are reported as the reciprocal of the highest serum dilution that resulted in ≥50% reduction in toxin activity. An increase of ≥4-fold over baseline was considered a positive response.
B cell memory
dmLT specific B memory responses were measured as previously described [25, 26]. Briefly, PBMCs were incubated with mitogen for five days to expand B memory cells that may be present [27]. Expanded cells were added to dmLT (5 μg/mL)-coated wells in multiScreen-HA plates (Millipore Sigma). dmLT specific and total IgG or IgA spot-forming cells (SFC) were developed and visualized using HRP-labeled anti-human IgA or IgG secondary antibodies and 3- Amino-9-ethylcarbazole (AEC) (Calbiochem, Millipore Sigma) substrate. For each subject, dmLT-specific IgA or IgG B memory spots were normalized by subtracting non-specific background SFC counts. B memory responses are reported as SFC/106 expanded cells or % of the corresponding total IgG or IgA spots. Subjects showing an increase of at least 0.05% in dmLT specific spots/total IgG or IgA above the pre-vaccination levels were considered responders.
T Cell-mediated Immunity
Th1 and Th2 dmLT-specific cytokine production in culture supernatants
Thawed and overnight rested PBMC (2×105 /well) from cohorts 4 and 5, were plated in duplicate wells of 96-well cell culture plates and cultured for 3 days in the presence of dmLT (5 μg/ml). Media only and Staphylococcus Enterotoxin B (SEB, 10 μg/ml) (Toxin Technology, Sarasota, FL) were used as no-antigen (background) and positive controls, respectively. Cytokines/chemokines were quantified in duplicate using the Proinflammatory Panel 1, Cytokine Panel 1, and Chemokine Panel I Human V-plex assay kits (Meso Scale Discovery, Bethesda, MD). An increase of ≥ 4-fold rise from baseline was considered a positive response.
Cytokine production by dmLT-specific CD4 and CD8 T memory cells
Thawed and overnight rested PBMC were stimulated with dmLT (5 μg/mL), culture media or SEB (10 μg/mL) as negative and positive controls, respectively. Surface and intracellular staining was performed following an overnight incubation with antigens using a 13-color panel that included flurochrome-labeled monoclonal antibodies against CD3, CD4, CD8, CD45RA, CD62L, CD107a, IFN-γ, TNF-α, MIP-1β, IL-2, IL-17 and CD69 as previously described [28,29]. Flow cytometry was performed using a customized LSRII flow cytometer (BD) and data were analyzed using WinList version 9 (Verity Software House, Topsham, ME, USA). The frequencies of cells producing cytokines (e.g., IFN-γ, TNF-α, MIP-1β, IL-2, IL-17) or expressing CD107a were measured in total live CD4+ or CD8+ cells, as well as in CD4+ or CD8+ Teffector/memory (TEM: CD45RA-CD62L-) subsets. Net responses in dmLT-stimulated samples were calculated by subtracting the responses in the media only controls. Post-vaccination minus corresponding pre-vaccination levels of ≥0.1% of Total or TEM cells for cytokine producing cells or those expressing CD107a were considered vaccine responders.
Statistics
Sample size were not based on power calculations but considered appropriate for a Phase 1 study. The details of number of subjects per group is summarized in Figure 1. To accommodate drop outs, cohorts 1–4 enrolled between 11 and 14 subjects, and the final cohort enrolled 16 subjects per route. With a total of 80 individuals, the absence of a dose-limiting AE provides for an exact upper 95% confidence bound of 4.5%.
Figure 1: Consort Flow Diagram.
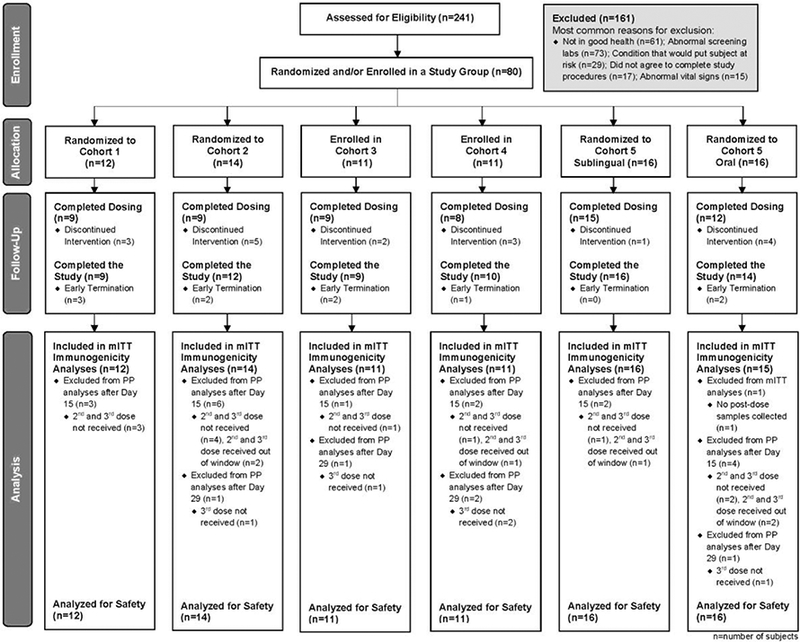
The analysis populations and subjects excluded are illustrated in this CONSORT flow diagram. mITT (modified intent to treat) population includes all subjects who received at least one dose of study product and contributed both pre- and at least one post-study vaccination samples for immunogenicity testing. The PP (per protocol) population includes all subjects in the mITT subset with the following exclusions: subjects found to be ineligible at baseline, second or third vaccination not received or received out of window. Receipt of non-study vaccines during the time frame was prohibited by the protocol.
Primary outcome measures included the occurrence of solicited AEs through day 8, vaccine-related unsolicited AEs through day 36, and facial nerve disturbance through day 104. The rates and exact 95% confidence intervals for the occurrence of AEs were calculated using the Clopper-Pearson method for binomial confidence intervals. Unsolicited AEs were coded by the Medical Dictionary for Regulatory Activities (MedDRA®) for preferred term and system organ class. The safety analysis population included all subjects who received at least one dose of study product.
Serum, fecal, and salivary antibody responses (by ELISA) and serum toxin neutralization antibody titers were analyzed by calculating the percent responders (≥4-fold rise from baseline) and the geometric mean titer (GMT). Hypothesis tests comparing cohort 5 – SL to cohort 5 – oral was conducted using two-sided Fisher’s exact tests for the proportion of responders or Wilcoxon rank-sum tests for the geometric mean fold rise (GMFR) at a 5% significance level.
The immunogenicity data are presented for the modified intent-to-treat (mITT) population. This includes all subjects who received at least one dose of study product and contributed both pre-and at least one post-study vaccination samples for immunogenicity testing for which valid results were reported. Results were similar for the per protocol (PP) population, which further excluded data subsequent to any major protocol deviations.
Results
To obtain proof-of-concept for SL administration of dmLT and compare this to oral administration, 241 subjects were screened to enroll a total of 80 subjects. The randomization or enrollment of subjects to various cohorts and completion of the study are shown in Figure 1. Of the 80 subjects enrolled, 12 subjects were enrolled in Cohort 1 (1 μg SL), 14 in Cohort 2 (5 μg SL), 11 in each of Cohorts 3 and 4 (25 and 50 μg, respectively), and 16 in each (SL or oral) of Cohort 5. In total, 80 subjects received dose 1, 67 subjects received 2 doses, and 62 subjects received all 3 doses. Overall, 71 subjects (89%) completed the final clinical follow-up visit on Day 85, and 70 subjects (88%) completed the final study contact by phone on day 210. The demographics for all subjects’ enrolled shows that gender, ethnicity, race and age were similar among cohorts (Table 1). Subjects excluded are illustrated in the CONSORT flow diagram (Figure 1).
Table 1:
Demographic and Baseline Characteristics of all enrolled subjects
| Characteristics | Cohorts, dmLT Doses and Routes | All Subjects |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5, 25 μg | |||
| 1 μg | 5 μg | 25 μg | 50 μg | Sublingual | Oral | ||
| n = 12 | n = 14 | n = 11 | n = 11 | n = 16 | n = 16 | n = 80 | |
| Gender, n (%) | |||||||
| Male | 7 (58) | 5 (36) | 5 (45) | 4 (36) | 6 (38) | 9 (56) | 36 (45) |
| Female | 5 (42) | 9 (64) | 6 (55) | 7 (64) | 10 (63) | 7 (44) | 44 (55) |
| Ethnicity, n (%) | |||||||
| Non-Hispanic | 12 (100) | 14 (100) | 10 (91) | 11 (100) | 14 (88) | 16 (100) | 77 (96) |
| Hispanic | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 1 (6) | 0 (0) | 2 (3) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 1 (1) |
| Race, n (%) | |||||||
| Black or African | |||||||
| American | 8 (67) | 5 (36) | 5 (45) | 6 (55) | 11 (69) | 10 (63) | 45 (56) |
| White | 3 (25) | 6 (43) | 5 (45) | 5 (45) | 5 (31) | 6 (38) | 30 (38) |
| Multiple | 1 (8) | 3 (21) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (5) |
| Asian | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Age, Years | |||||||
| Mean | 29.2 | 26.9 | 32.5 | 26.7 | 25.8 | 29.9 | 28.4 |
| SD | 8.3 | 6.3 | 6.8 | 6.8 | 5.4 | 7.5 | 7.0 |
N=Number of enrolled subjects.
Cohorts 1–4 received sublingual dmLT
Other races where the numbers were 0 are not included in this table
Safety
The vaccine appeared to be safe at all SL doses evaluated (Table 2). Solicited AEs occurred infrequently and were mostly mild. The most prevalent systemic symptom was abdominal pain, reported in 12 of 64 (19%) subjects after a SL dose, and the only solicited local reactogenicity symptoms were oral local reactions reported in 6 (9%) subjects. After a SL dose, diarrhea developed in only one subject (5 μg dose) and vomiting in only three (one each 5, 50, and 25 μg SL dose). The only severe symptom noted was vomiting in one subject (50 μg) on day 8 after the third vaccination. Moderate abdominal pain was reported in six subjects and moderate decreased appetite/anorexia in five. No consistent increase in the frequency of AEs was noted by the dose number or dose amount. Results following the oral doses (25 μg) were similar.
Table 2:
Subjects experiencing any solicited symptoms following administration of all vaccine doses
| Cohorts, dmLT Dose and Routes; % with symptoms (95% CI) | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5, 25 μg | ||
| 1 μg | 5 μg | 25 μg | 50 μg | Sublingual | Oral | |
| Symptoms | n =12 | n =14 | n =11 | n =11 | n = 16 | n = 16 |
| Any Systemic | 17 | 29 | 27 | 9 | 25 | 31 |
| Symptom | (2, 48) | (8, 58) | (6, 61) | (0, 41) | (7, 52) | (11, 59) |
| Fever | 0 | 7 | 0 | 0 | 0 | 0 |
| (0, 26) | (0, 34) | (0, 28) | (0, 28) | (0, 21) | (0, 21) | |
| Diarrhea | 0 | 7 | 0 | 0 | 0 | 0 |
| (0, 26) | (0, 34) | (0, 28) | (0, 28) | (0, 21) | (0, 21) | |
| Abdominal Pain | 8 | 21 | 27 | 9 | 25 | 25 |
| (0, 38) | (5, 51) | (6, 61) | (0, 41) | (7, 52) | (7, 52) | |
| Anorexia | 17 | 14 | 18 | 9 | 19 | 6 |
| (2, 48) | (2, 43) | (2, 52) | (0, 41) | (4, 46) | (0, 30) | |
| Vomiting | 0 | 7 | 0 | 9 | 6 | 6 |
| (0, 26) | (0, 34) | (0, 28) | (0, 41) | (0, 30) | (0, 30) | |
| Any Local Symptom | 8 | 14 | 27 | 0 | 0 | 6 |
| (8, 38) | (2, 34) | (6, 61) | (0, 28) | (0, 21) | (0, 30) | |
| Oral Local Reactions | 8 | 14 | 27 | 0 | 0 | 6 |
| (8, 38) | (2, 43) | (6, 61) | (0, 28) | (0, 21) | (0, 30) | |
| Any Symptom | 17 | 36 | 36 | 9 | 25 | 31 |
| (2, 48) | (13, 65) | (11, 69) | (0, 41) | (7,52) | (11,59) | |
Cohorts 1–4 received sublingual dmLT
Denominator for percentages is the number of subjects in the safety analysis population (all subjects who received at least one dose of study product). Numbers in parenthesis represent 95% CI based on an exact binomial distribution (Clopper-Pearson)
Unsolicited AEs were also relatively uncommon following SL immunization. Seventeen (17) subjects (27%) reported any vaccine-related, unsolicited AE within 36 days following any SL dose. AEs did not appear to increase with subsequent doses nor were increases noted with increasing dose levels. The most common MedDRA System Organ Class (SOC) of AEs was “Gastrointestinal Disorders” (14 subjects). Of these, the most frequent were those classified in the MedDRA Preferred Term, “Flatulence” (n=5). No subjects reported facial nerve disturbance.
Abnormal laboratory values were observed among 43 of the 64 subjects receiving SL dosing during the course of the study. All were mild or moderate and self-correcting and were distributed among many tests.
Immunogenicity
The magnitude of the serum anti-dmLT IgA and IgG titers are summarized in Figure 2, and Table 3 shows the responder rates following vaccination. Responses were modest after any of the SL immunizations. Titers peaked after the third immunization but the percent of subjects with 4fold increases was at best 38% for both IgA and IgG in the mITT analysis at any time (Table 3). If only subjects who received all 3 doses were evaluated 43% developed a 4-fold rise for IgA and IgG. Responses tended to be higher following larger doses of 25 to 50 μg. Although the frequency of the responders was modest, the average fold rise among responders in 25 or 50 μg groups following SL delivery for IgA and IgG were 10- and 18-fold, respectively (data not shown). Comparison of the SL vs. oral route in cohort 5 tended to favor the oral route but none of the comparisons of the percent with 4-fold rises were significant (Table 3). For serum anti-dmLT IgG, GMFRs were significantly higher in the oral dmLT group compared to the sublingual group at days 36 and 85 but only for the PP population (p=0.028 and p=0.032, respectively, data not shown).
Figure 2: Serum anti-dmLT IgA (A) and IgG (B) titers as measured by ELISA, mITT population.
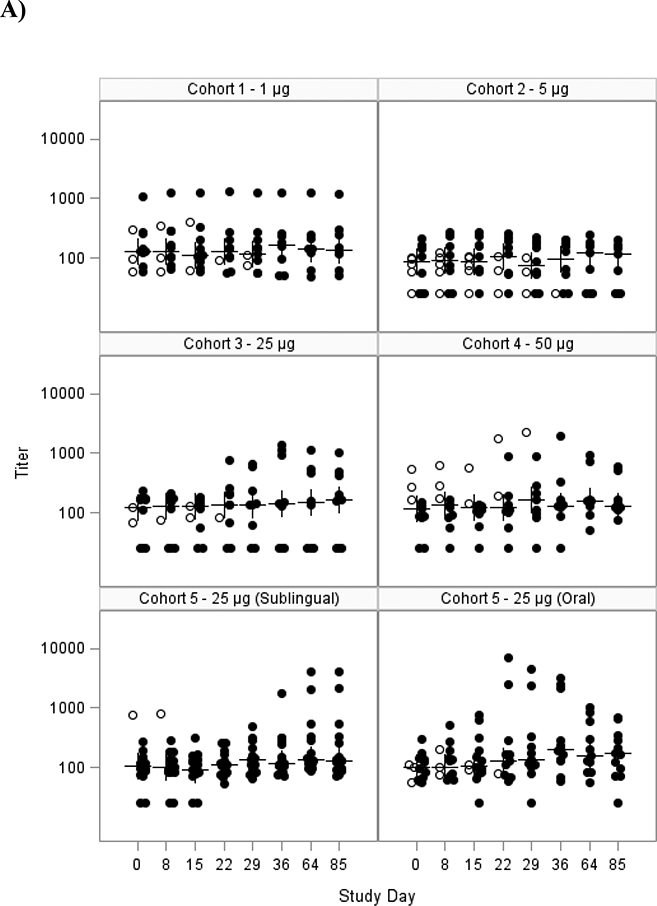
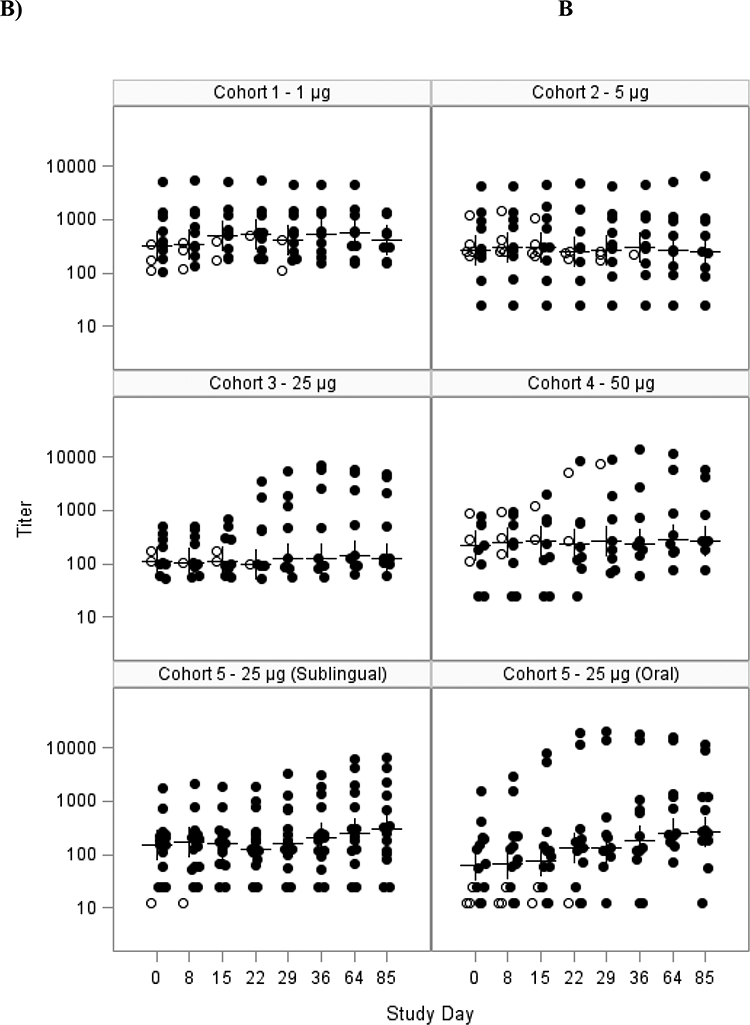
Scatter plot showing the A) log10 serum IgA antibody response to dmLT for each subject in the mITT population for each cohort and B) log10 serum IgG antibody response. Cohorts 1–4 received SL dmLT. Cohort 5 was randomized to receive SL or Oral dmLT.
The closed circles are individuals who received all 3 doses; the open circles are individuals that received <3 doses. The crosses represent the median at each time point. Subjects were vaccinated on days 0, 15 and 29.
Table 3:
Serum Anti-dmLT IgA and IgG Seroconversion (4-fold rise)
| Cohorts, dmLT Doses and Routes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Days post vaccination |
4-fold risea | 1 | 2 | 3 | 4 | 5, 25 μg | p value* | |
| 1 μg | 5 μg | 25 μg | 50 μg | Sublingual | Oral | |||
| Day 8 | n | 12 | 14 | 10 | 11 | 16 | 15 | |
| IgA | 0 (0, 26)b | 0 (0, 23) | 0 (0, 31) | 0 (0, 28) | 0 (0, 21) | 7 (0, 32) | 0.484 | |
| IgG | 0 (0, 26) | 0 (0, 23) | 0 (0, 31) | 0 (0, 28) | 0 (0, 21) | 0 (0, 22) | ---- | |
| Neutralization | 0 (0, 26) | 0 (0, 23) | 0 (0, 31) | 0 (0, 28) | 0 (0, 21) | 7 (0, 32) | 0.484 | |
| Day 15 | n | 11 | 14 | 11 | 10 | 15 | 14 | |
| IgA | 0 (0, 28) | 0 (0, 23) | 0 (0, 28) | 0 (0, 31) | 0 (0, 22) | 14 (2, 43) | 0.224 | |
| IgG | 0 (0, 28) | 0 (0, 23) | 0 (0, 28) | 0 (0, 31) | 0 (0, 22) | 14 (2, 43) | 0.224 | |
| Neutralization | 0 (0, 28) | 0 (0, 23) | 0 (0, 28) | 0 (0, 31) | 0 (0, 22) | 14 (2, 43) | 0.224 | |
| Day 22 | n | 10 | 11 | 10 | 10 | 15 | 13 | |
| IgA | 0 (0, 31) | 0 (0, 28) | 10 (0, 45) | 10 (0, 45) | 0 (0, 22) | 15 (2, 45) | 0.206 | |
| IgG | 0 (0, 31) | 0 (0, 28) | 20 (3, 56) | 20 (3, 56) | 13 (2, 40) | 23 (5, 54) | 0.639 | |
| Neutralization | 10 (0, 45) | 0 (0, 28) | 10 (0, 45) | 0 (0, 31) | 7 (0, 32) | 15 (2, 45) | 0.583 | |
| Day 29 | n | 11 | 12 | 9 | 9 | 15 | 11 | |
| IgA | 0 (0, 28) | 0 (0, 26) | 0 (0, 34) | 22 (3, 60) | 13 (2, 40) | 18 (2, 52) | 1.000 | |
| IgG | 0 (0, 28) | 0 (0, 26) | 33 (7, 70) | 22 (3, 60) | 13 (2, 40) | 27 (6, 61) | 0.620 | |
| Neutralization | 0 (0, 28) | 0 (0, 26) | 11 (0, 48) | 11 (0, 48) | 7 (0, 32) | 27 (6, 61) | 0.279 | |
| Day 36 | n | 9 | 10 | 9 | 8 | 15 | 12 | |
| IgA | 0 (0, 34) | 0 (0, 31) | 33 (7, 70) | 25 (3, 65) | 13 (2, 40) | 33 (10, 65) | 0.357 | |
| IgG | 0 (0, 34) | 0 (0, 31) | 33 (7, 70) | 38 (9, 76) | 13 (2, 40) | 42 (15, 72) | 0.185 | |
| Neutralization | 0 (0, 34) | 0 (0, 31) | 22 (3, 60) | 13 (0, 53) | 7 (0, 32) | 25 (5, 57) | 0.294 | |
| Day 64 | n | 9 | 9 | 9 | 8 | 14 | 11 | |
| IgA | 0 (0, 34) | 0 (0, 34) | 11 (0, 48) | 38 (9, 76) | 29 (8, 58) | 27 (6, 61) | 1.000 | |
| IgG | 0 (0, 34) | 0 (0, 34) | 33 (7, 70) | 38 (9, 76) | 29 (8, 58) | 45 (17, 77) | 0.434 | |
| Neutralization | 0 (0, 34) | 0 (0, 34) | 33 (7, 70) | 25 (3, 65) | 14 (2, 43) | 27 (6, 61) | 0.623 | |
| Day 85 | n | 8 | 9 | 9 | 8 | 15 | 12 | |
| IgA | 0 (0, 37) | 0 (0, 34) | 11 (0, 48) | 38 (9, 76) | 13 (2, 40) | 25 (5, 57) | 0.628 | |
| IgG | 0 (0, 37) | 0 (0, 34) | 33 (7, 70) | 38 (9, 76) | 27 (8, 55) | 50 (21, 79) | 0.257 | |
| Neutralization | 0 (0, 37) | 0 (0, 34) | 33 (7, 70) | 25 (3, 65) | 13 (2, 40) | 25 (5, 57) | 0.628 | |
| Any Time | n | 12 | 14 | 11 | 11 | 16 | 15 | |
| IgA | 0 (0, 26) | 0 (0, 23) | 27 (6, 61) | 36 (11, 69) | 25 (7, 52) | 33 (12, 62) | 0.704 | |
| IgG | 0 (0, 26) | 0 (0, 23) | 27 (6, 61) | 36 (11, 69) | 25 (7, 52) | 40 (16, 68) | 0.458 | |
| Neutralization | 8 (0, 38) | 0 (0, 23) | 27 (6, 61) | 27 (6, 61) | 13 (2, 38) | 27 (8, 55) | 0.394 | |
Number of subjects (n) and results correspond to mITT population. SL: sublingual dmLT; O: oral dmLT.
Represents the percentage of subjects with at least a 4-fold increase compared to pre-dose 1.
Numbers in parenthesis represent 95% CI based on an exact binomial distribution (Clopper-Pearson)
Fisher’s exact test comparing 4-Fold Rise between Cohort 5 – 25μg SL and Cohort 5 – 25μg Oral
Neutralizing antibody responses followed a similar variable response pattern even at the highest dose (Table 3, Figure 3). The oral dmLT group had a significantly higher GMFR (2.7; 95% CI: 1.0, 7.3) compared to the sublingual dmLT group (1.1; 95% CI: 0.9, 1.4) at day 29 (p=0.028), however there were no significant differences between groups with respect to the percentage of subjects with a 4-fold rise or higher (Table 3).
Figure 3: Serum LT-neutralization GMT, mITT Population.
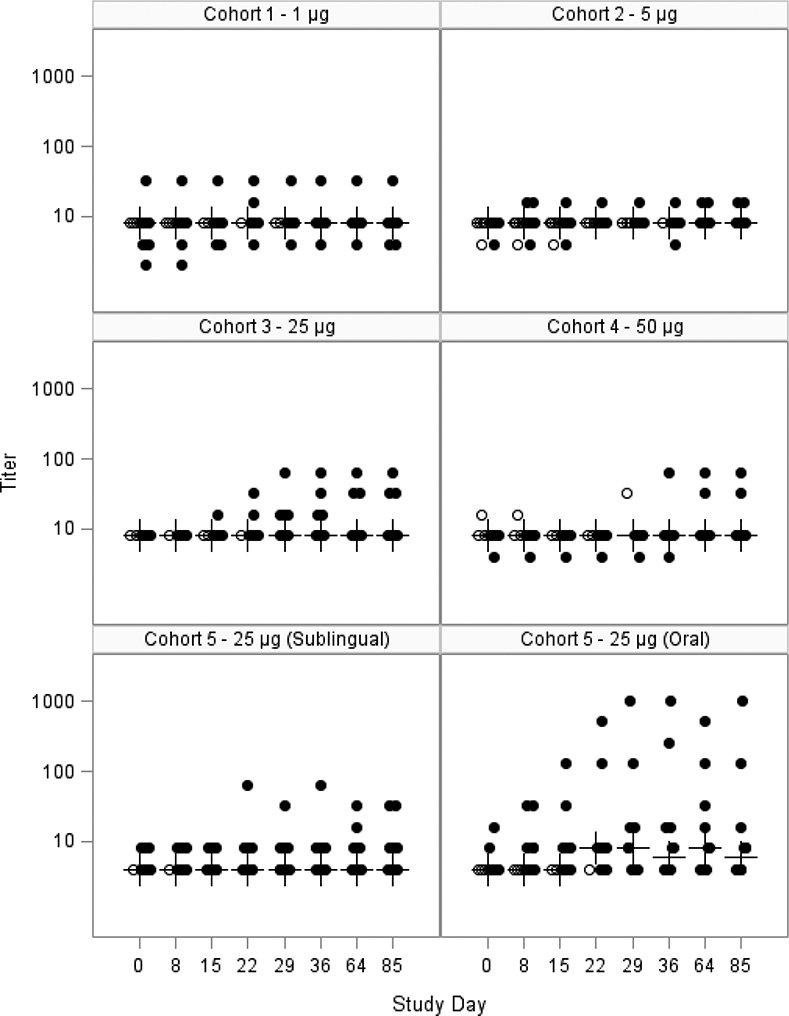
Scatter plot showing the log10 serum neutralizing antibody response to dmLT for each subject in the mITT population for each cohort. The closed circles are individuals who received all 3 doses; the open circles are individuals that received <3 doses. The crosses represent the median at each time point. Subjects were vaccinated on days 0, 15 and 29.
The number of IgA and IgG ASC in circulation increased after immunization but the levels were of mild to moderate magnitude (Table 4). The highest percentage of IgA or IgG responders was 43% of subjects on day 34 (not shown). Similar to the anti-dmLT serum antibody response, the highest ASC levels were seen in the groups that received the two highest vaccine doses, especially the 25 μg dose and after 3 immunizations (20.3±28.6 IgA ASC/106 PBMC). A higher percentage of subjects in the oral dmLT group were IgA ASC responders at day 36 and overall compared to the SL dmLT group. No significant differences between oral and SL dmLT groups were observed for IgG ASC responses. Similar findings were observed for the comparison of ALS (Table 4); significantly higher responses were found for the GMFR for IgA at day 34 (p=0.012). Due to the mild to moderate induction of dmLT-specific ASC responses, studies directed to evaluate integrin α4β7 receptors in ASCs were inconclusive.
Table 4:
Percent with response in antibody secreting cell or antibodies in lymphocyte supernatants at select time points
| IgA | IgG | |||||||
|---|---|---|---|---|---|---|---|---|
| Dose | Day 8 | Day 22 | Day 36 | Any Time | Day 8 | Day 22 | Day 36 | Any Time |
| ASC | ||||||||
| Cohort 3 – 25 μg | 0 (0, 31) | 10 (0, 45) | 33 (7, 70) | 27 (6, 61) | 0 (0, 31) | 0 (0, 31) | 33 (7, 70) | 27 (6, 61) |
| Cohort 4 – 50 μg | 0 (0, 28) | 0 (0, 31) | 0 (0, 37) | 9 (0, 41) | 0 (0, 28) | 0 (0, 31) | 0 (0, 37) | 9 (0, 41) |
| Cohort 5 – 25 μg (Sublingual) | 0 (0, 21) | 0 (0, 22) | 0 (0, 22) | 0 (0, 21) | 0 (0, 21) | 0 (0, 22) | 0 (0, 22) | 0 (0, 21) |
| Cohort 5 – 25 μg (Oral) | 7 (0, 32) | 15 (2, 45) | 33 (10, 65) | 27 (8, 55) | 13 (2, 40) | 15 (2, 45) | 0 (0, 26) | 13 (2, 40) |
| ALS | ||||||||
| Cohort 3 – 25 μg | 0 (0, 31) | 30 (7, 65) | 33 (7, 70) | 27 (6, 61) | 20 (3, 56) | 30 (7, 65) | 44 (14, 79) | 36 (11, 69) |
| Cohort 4 – 50 μg | 0 (0, 28) | 20 (3, 56) | 13 (0, 53) | 18 (2, 52) | 9 (0, 41) | 20 (3, 56) | 25 (3, 65) | 27 (6, 61) |
| Cohort 5 – 25 μg (Sublingual) | 0 (0, 21) | 0 (0, 22) | 7 (0, 32) | 6 (0, 30) | 0 (0, 21) | 7 (0, 32) | 13 (2, 40) | 13 (2, 38) |
| Cohort 5 – 25 μg (Oral) | 7 (0, 32) | 15 (2, 45) | 17 (2, 48) | 40 (16, 68) | 13 (2, 40) | 15 (2, 45) | 17 (2, 48) | 27 (8, 55) |
Note: A responder is defined as >8 ASC / 106 PBMC for ASC, or having at least a 2-fold rise in antibody compared to pre-dose 1 for ALS. 95% CIs are shown in parenthesis
Only data for the higher doses at one week post each immunization are shown. Responses were minimal at the lower doses.
Mucosal antibody responses were rarely detected in either the stool or saliva. In general, responses were somewhat higher following oral immunization with significant differences noted for the GMFR in stool IgA at days 36 (1.39 vs. 0.66, p=0.033) and 64 (1.62 vs. 0.66, p=0.014). However, the highest percent with 4-fold increases was only 20% detected in the stool of oral vaccine recipients at day 64. Similarly, B memory cell responses including memory IgA and IgG were infrequent for either route of immunization at any time.
Post-vaccination increases in cytokine production by PBMC in culture supernatants following stimulation with dmLT were inconsistent and of low magnitude. However, the oral group exhibited significantly higher response rates for several cytokines at multiple time points compared to the SL group.
The frequencies of circulating dmLT-specific total and TEM CD4+ or CD8+ cells that produced cytokine or expressed CD107a (functional cells) were also measured. Highest post-vaccination increases (>0.1 over pre-vaccination levels) were observed in CD8+ and CD4+ MIP-1β+ TEM cells in 75% (6 out of 8) and 38% (3 out of 8), respectively, in cohort 4 (50 μg/mL dose). Very low proportions of volunteers (0–38%) showed responses with other cytokine and/or CD107a expressing CD4+ or CD8+ cells in cohort 4, or with any cytokine in cohort 5. No significant differences were observed comparing oral to SL subjects.
Discussion
One of the principal ETEC virulence factors, heat-labile toxin (LT), has been studied as a potential vaccine antigen, as well as an adjuvant to induce mucosal immune responses. An ETEC vaccine candidate consisting of native LT delivered transcutaneously with a dermal patch was found to be immunogenic, and demonstrated varying levels of efficacy in Phase 2b and Phase 3 field efficacy studies in travelers to Guatemala and Mexico, with the most significant level of protection seen against ETEC strains producing only LT toxin in the Phase 3 trial [30]. However, a safer, non-toxic mutant of LT may provide more options for vaccine formulation, dose and delivery [31]. Further, a mutant LT based vaccine may have the significant advantage of being able to be given orally, a more practical approach for vaccine delivery to infants and young children in low-resource settings who are at high risk for ETEC-associated morbidity and mortality. Therefore, several attempts to modify the protein so that it maintains its immunogenicity and adjuvant properties without toxicity were made [32–35]. The single mutant (LT(R192G) seemed to retain their immunogenicity but were not fully attenuated and appeared to induce a moderate self-limiting diarrhea primarily following the first vaccine doses in 15–25% of subjects given 25 μg of LT(R192G) [7, 36]. Therefore, double mutants were developed and evaluated here [4, 9].
We studied the SL route because animal studies have shown that the SL route was not only well tolerated, but also induces serum and mucosal immune responses on a broad range of mucosal surfaces, including the respiratory, gastrointestinal, and urogenital tracts comparable or better than other routes [17, 37–40]. After selection of the best SL dose we compared the SL route to oral.
The initial evaluations were performed as a dose escalation study of concentrations including 1 μg, 5 μg, 25 μg and 50 μg administered by the SL route. All dose levels were safe and well tolerated. GI toxicity was infrequently observed even the highest doses, with mild to moderate abdominal pain the most common symptom reported (9–27% of high dose recipients). Vomiting was seen in only three SL recipients and diarrhea in only one SL recipient. However, the vaccine was only moderately immunogenic by the SL route. Anti-dmLT IgA and IgG responses peaked after the third SL immunization but GMFR increases were never greater than 2.5 for IgA and 3.4 for IgG. Similarly, the percent with 4-fold increases was at best 43% for IgA and 43% for IgG in the PP population at any time. Responses tended to be higher following doses of 25 to 50 μg. Similar results for the neutralizing antibody response were found. Mucosal antibodies were rarely detected in either the stool or saliva. Comparison of the SL vs. oral route in Cohort 5 tended to favor the oral route for both systemic and mucosal responses. Cell mediated immunity including multiple cytokine responses were only rarely detected.
In a previous trial, El-Kamary et al. [11] reported that after a single oral dose of 50 μg, 67% of volunteers developed a 4-fold rise in serum anti-dmLT IgA and 58% a 4-fold rise in anti-dmLT IgG. Responses to 25 or 100 μg doses were not as high. In the study presented here none of the participants developed a 4-fold rise of either IgA or IgG after a single SL dose (1 to 50 μg). In the comparator (25 μg) oral dose group, a 4-fold IgA and IgG response was detected in 14% of recipients after a single oral immunization. This is similar to the 16.7% response in the previous study after a single 25 μg dose. Oral dosing with 50 μg was not performed in the present study, as we used the same (25 μg) dose for both oral and SL vaccination.
Others have reported lower immunogenicity of human papillomavirus vaccine given to healthy female volunteers by SL as compared to parenteral immunization [41]. In this study, SL vaccination failed to elicit mucosal immunity (i.e., virus neutralizing activity and ASC). These results as well as ours contradict numerous murine studies, in which SL immunization was shown to be highly effective at inducing both systemic and mucosal immunity [42] even outperforming other routes of vaccination [21].
One potential limitation of sublingual delivery in humans is the lack of control in dose and duration of administration compared to typical vaccine preparations and delivery methods where antigen and adjuvant are mixed in saline and administered and given orally or parenterally. In the absence of mucoadhesive agents in such formulations, there is a lower retention at the site (involuntary swallowing) resulting in potential loss of antigen/adjuvant dose. In addition, there is potential dilution by saliva (saliva secretion) and antigen degradation by salivary enzymes which further limits the amount/dose available for sublingual absorption.
However, despite these limitations, dmLT-specific immunity was produced in ~40% of the volunteers, who developed 4-fold rises in serum IgA and/or IgG. A dose response trend in antibody titers and IgA and/or IgG B memory responses were observed in subjects who received 25 and 50 μg of dmLT by the SL route. The majority of subjects mounting anti-dmLT serum antibody responses after receiving sublingual doses of 25ug also responded with anti-dmLT mucosal or functional antibody responses as measured by saliva, ASC, ALS, fecal IgA or toxin neutralization assays indicating that when responses were induced they tended to be robust (data not shown). Further, a larger proportion of volunteers with dmLT-neutralizing antibodies above detection levels at baseline (28% vs. 8% without detectable antibody) seroconverted after SL vaccination, suggesting that SL immunization might be useful in prime-boost combinations, as proposed by others (41). Together, these observations provide proof of concept for SL vaccine delivery in humans. Future studies should consider evaluating higher doses delivered via a mucoadhesive gel carrier. This might serve to enhance adhesion of dmLT to the sublingual mucosa and to facilitate more efficient capture by the local immune system [43–45].
In summary, the dmLT vaccine evaluated here was safe and moderately immunogenic at doses up to 50 μg when administered by the SL or oral route. Although responses directed against LT were not acceptable for an ETEC vaccine the possibility for use as an adjuvant remains. To date, dmLT has been shown to enhance the antibody response to co-administered ETEC colonization antigen in both Swedish and Bangladeshi adults and studies evaluating it as a parenteral adjuvant with an ETEC subunit vaccine are in progress [46, 47]. In considering these results it should be noted that the immunogenicity of dmLT given by the SL route may have been underestimated since the vaccine antigen was not formulated in a way to prevent degradation and improve its contact time with and persistence at the sublingual surface.
Highlights.
The public health burden of Enterotoxigenic Escherichia coli (ETEC) is high but no vaccine is specifically approved to prevent ETEC infections.
This dose escalating clinical trial advances our knowledge of ETEC vaccines. It showed that the formulation of the dmLT vaccine evaluated here, LTR192G/L211A, was safe, achieving the primary objective of the study but only moderately immunogenic at doses up to 50 μg when administered by the sublingual or oral route.
Although responses directed against LT were not acceptable for an ETEC vaccine the possibility for use as an oral adjuvant remains.
It should also be noted that the immunogenicity of dmLT given by the sublingual route may be improved by decreasing degradation and increasing persistence at the sublingual surface.
Acknowledgements
This work was supported by funds from the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, under contracts HHSN272200800006C (Cincinnati Children’s Hospital), HHSN272200800057C (University of Maryland, Baltimore); and HHSN272200800013C (Emmes Corporation) and PATH under agreement OPP1112376.
We especially want to thank Gabriele Feolo, Clinical project Manager at NIAID and Amy Cline, Michelle Dickey and Tara Foltz for coordinating this study at CCHMC; Mardi Reymann (Applied Immunology Lab) for performing the serological and ASC assays at the University Maryland and Heather Wenzel and Jessica White of PATH for coordinating dmLT stability testing and dose verification testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
This article was prepared while Amanda D. Buskirk, Ph.D. was employed at the University of Maryland School of Medicine Center for Vaccine Development and Global Health. The opinions expressed in this article are those of the authors and do not reflect the view of the Food and Drug Administration, the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, the Department of Health and Human Services, or the United States Government
References
- [1].Walker RI, Steele D, Aguado T, Ad Hoc ETEC. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine. 2007;25:2545–66. [DOI] [PubMed] [Google Scholar]
- [2].Dickinson BL, Clements JD. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheng E, Cardenas-Freytag L, Clements JD. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine. 1999;18:38–49. [DOI] [PubMed] [Google Scholar]
- [4].Norton EB, Lawson LB, Freytag LC, Clements JD. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol. 2011;18:546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, et al. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled Phase I study. Vaccine. 2014;32:7077–84. [DOI] [PubMed] [Google Scholar]
- [6].Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–81. [DOI] [PubMed] [Google Scholar]
- [7].Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69:3581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tribble D, Baqar S, Thompson S. Development of a human vaccine In: ISCM N, Blaser M, editors. Camppylobacter. 3rd ed Washington, DC: ASM Press; 2008. p. 429–44. [Google Scholar]
- [9].Summerton NA, Welch RW, Bondoc L, Yang HH, Pleune B, Ramachandran N, et al. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin. Vaccine. 2010;28:1404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ahren CM, Svennerholm AM. Synergistic protective effect of antibodies against Escherichia coli enterotoxin and colonization factor antigens. Infect Immun. 1982;38:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].El-Kamary SS, Cohen MB, Bourgeois AL, Van De Verg L, Bauers N, Reymann M, et al. Safety and immunogenicity of a single oral dose of recombinant double mutant heat-labile toxin derived from enterotoxigenic Escherichia coli. Clin Vaccine Immunol. 2013;20:1764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Torrell JM, Aumatell CM, Ramos SM, Mestre LG, Salas CM. Reduction of travellers’ diarrhoea by WC/rBS oral cholera vaccine in young, high-risk travellers. Vaccine. 2009;27:4074–7. [DOI] [PubMed] [Google Scholar]
- [13].Peltola H, Siitonen A, Kyronseppa H, Simula I, Mattila L, Oksanen P, et al. Prevention of travellers’ diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet. 1991;338:1285–9. [DOI] [PubMed] [Google Scholar]
- [14].Clemens JD, Sack DA, Harris JR, Chakraborty J, Neogy PK, Stanton B, et al. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J Infect Dis. 1988;158:372–7. [DOI] [PubMed] [Google Scholar]
- [15].Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind-Gerson J, Figueroa JF, et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet. 2008;371:2019–25. [DOI] [PubMed] [Google Scholar]
- [16].Ellingsworth L, et al. Transcutaneous immunization and the travelers’ diarrhea vaccine system: A Phase III pivotal efficacy study. 6th International Conference on Vaccines for Enteric Diseases Cannes, France 2011. [Google Scholar]
- [17].Amuguni H, Lee S, Kerstein K, Brown D, Belitsky B, Herrmann J, et al. Sublingual immunization with an engineered Bacillus subtilis strain expressing tetanus toxin fragment C induces systemic and mucosal immune responses in piglets. Microbes Infect. 2012;14:447–56. [DOI] [PubMed] [Google Scholar]
- [18].Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23:1804–13. [DOI] [PubMed] [Google Scholar]
- [19].Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–53. [DOI] [PubMed] [Google Scholar]
- [20].Sjokvist Ottsjo L, Flach CF, Clements J, Holmgren J, Raghavan S. A double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect Immun. 2013;81:1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Luo Q, Vickers TJ, Fleckenstein JM. Immunogenicity and Protective Efficacy against Enterotoxigenic Escherichia coli Colonization following Intradermal, Sublingual, or Oral Vaccination with EtpA Adhesin. Clin Vaccine Immunol. 2016;23:628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tacket CO, Pasetti MF, Edelman R, Howard JA, Streatfield S. Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine. 2004;22:4385–9. [DOI] [PubMed] [Google Scholar]
- [23].Toapanta FR, Simon JK, Barry EM, Pasetti MF, Levine MM, Kotloff KL, et al. Gut-Homing Conventional Plasmablasts and CD27(−) Plasmablasts Elicited after a Short Time of Exposure to an Oral Live-Attenuated Shigella Vaccine Candidate in Humans. Front Immunol. 2014;5:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Glenn GM, Villar CP, Flyer DC, Bourgeois AL, McKenzie R, Lavker RM, et al. Safety and immunogenicity of an enterotoxigenic Escherichia coli vaccine patch containing heat-labile toxin: use of skin pretreatment to disrupt the stratum corneum. Infect Immun. 2007;75:2163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Simon JK, Wahid R, Maciel M Jr., Picking WL, Kotloff KL, Levine MM, et al. Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates. Vaccine. 2009;27:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wahid R, Pasetti MF, Maciel M Jr., Simon JK, Tacket CO, Levine MM, et al. Oral priming with Salmonella Typhi vaccine strain CVD 909 followed by parenteral boost with the S. Typhi Vi capsular polysaccharide vaccine induces CD27+IgD-S. Typhi-specific IgA and IgG B memory cells in humans. Clin Immunol. 2011;138:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. JImmunolMethods. 2004;286:111–22. [DOI] [PubMed] [Google Scholar]
- [28].Wahid R, Fresnay S, Levine MM, Sztein MB. Immunization with Ty21a live oral typhoid vaccine elicits crossreactive multifunctional CD8+ T-cell responses against Salmonella enterica serovar Typhi, S. Paratyphi A, and S. Paratyphi B in humans. Mucosal Immunol. 2015;8:1349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wahid R, Fresnay S, Levine MM, Sztein MB. Cross-reactive multifunctional CD4+ T cell responses against Salmonella enterica serovars Typhi, Paratyphi A and Paratyphi B in humans following immunization with live oral typhoid vaccine Ty21a. Clin Immunol. 2016;173:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Behrens RH, Cramer JP, Jelinek T, Shaw H, von Sonnenburg F, Wilbraham D, et al. Efficacy and safety of a patch vaccine containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase 3, randomised, double-blind, placebo-controlled field trial in travellers from Europe to Mexico and Guatemala. Lancet Infect Dis. 2014;14:197–204. [DOI] [PubMed] [Google Scholar]
- [31].Zhang W, Sack DA. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev Vaccines. 2012;11:677–94. [DOI] [PubMed] [Google Scholar]
- [32].da Hora VP, Conceicao FR, Dellagostin OA, Doolan DL. Non-toxic derivatives of LT as potent adjuvants. Vaccine. 2011;29:1538–44. [DOI] [PubMed] [Google Scholar]
- [33].Peppoloni S, Ruggiero P, Contorni M, Morandi M, Pizza M, Rappuoli R, et al. Mutants of the Escherichia coli heat-labile enterotoxin as safe and strong adjuvants for intranasal delivery of vaccines. Expert Rev Vaccines. 2003;2:285–93. [DOI] [PubMed] [Google Scholar]
- [34].Ghiara P, Rossi M, Marchetti M, Di Tommaso A, Vindigni C, Ciampolini F, et al. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Marchetti M, Rossi M, Giannelli V, Giuliani MM, Pizza M, Censini S, et al. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–7. [DOI] [PubMed] [Google Scholar]
- [36].Lapa JA, Sincock SA, Ananthakrishnan M, Porter CK, Cassels FJ, Brinkley C, et al. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat-labile enterotoxin with mutation R192G. Clin Vaccine Immunol. 2008;15:1222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sjokvist Ottsjo L, Jeverstam F, Yrlid L, Wenzel AU, Walduck AK, Raghavan S. Induction of mucosal immune responses against Helicobacter pylori infection after sublingual and intragastric route of immunization. Immunology. 2017;150:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gallorini S, Taccone M, Bonci A, Nardelli F, Casini D, Bonificio A, et al. Sublingual immunization with a subunit influenza vaccine elicits comparable systemic immune response as intramuscular immunization, but also induces local IgA and TH17 responses. Vaccine. 2014;32:2382–8. [DOI] [PubMed] [Google Scholar]
- [39].Shim BS, Choi Y, Cheon IS, Song MK. Sublingual delivery of vaccines for the induction of mucosal immunity. Immune Netw. 2013;13:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, et al. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25:8598–610. [DOI] [PubMed] [Google Scholar]
- [41].Huo Z, Bissett SL, Giemza R, Beddows S, Oeser C, Lewis DJ. Systemic and mucosal immune responses to sublingual or intramuscular human papilloma virus antigens in healthy female volunteers. PLoS One. 2012;7:e33736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].White JA, Blum JS, Hosken NA, Marshak JO, Duncan L, Zhu C, et al. Serum and mucosal antibody responses to inactivated polio vaccine after sublingual immunization using a thermoresponsive gel delivery system. Hum Vaccin Immunother. 2014;10:3611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP. Buccal and sublingual vaccine delivery. J Control Release. 2014;190:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Amorij JP, Kersten GF, Saluja V, Tonnis WF, Hinrichs WL, Slutter B, et al. Towards tailored vaccine delivery: needs, challenges and perspectives. J Control Release. 2012;161:363–76. [DOI] [PubMed] [Google Scholar]
- [45].Lal M, White J, Changcheng Z. Preparing an Adjuvanted Thermoresponsive Gel Formulation for Sublingual Vaccination Methods in Molecular Biology: Humana Press; 2016. p. 153–63. [DOI] [PubMed] [Google Scholar]
- [46].Akhtar M, Chowdhury MI, Bhuiyan RR et al. E. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind randomized placebo-controlled Phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Submitted Vaccine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bourgeois AL, Wierzba TF, Walker RI. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. 2016;34:2880–6. [DOI] [PubMed] [Google Scholar]


