Figure 6.
Mechanical Competition Eliminates Cells through Compaction-Driven ERK Downregulation
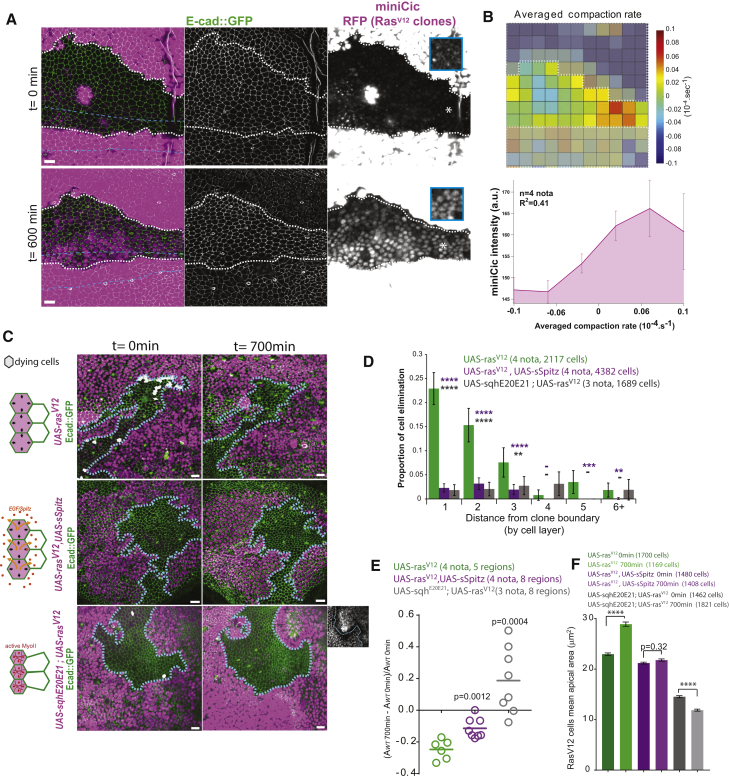
(A) Snapshots (0 and 600 min; local z-projections) of a live pupal notum; endo-Ecad::GFP (green) miniCic (magenta); induction of RasV12 in clones (RFP, strong magenta, white dashed lines); midline: blue dotted lines; blue rectangles: single plane miniCic signal from asterisk regions. Scale bars represent 10 μm.
(B) Top: map of the averaged compaction rate (PIV, white dashed lines: clone boundaries). Red: compaction; blue: stretching. Bottom: averaged local miniCic intensity at 600 min for a given local compaction rate is shown (4 nota). R2: Pearson correlation coefficient. Error bars indicate SEM.
(C) z-projections (0 min and after 700 min) of live pupal nota expressing endo-Ecad::GFP (green) upon conditional induction of RasV12, RasV12 and sSpitz (Drosophila EGF), or RasV12 and sqhE20E21 (constitutively active Myosin II regulatory light chain) in clones (RFP, magenta, blue dashed lines: contours). Dying cells are shown in white. Left schemes: expected deformations of the cells are shown (clone, purple). Bottom right inset: E-cad::GFP in Ras and active MyoII clones are shown. Scale bars represent 10 μm.
(D) Probability of cell death for a given distance to clone boundaries (in cell rows). ∗∗∗∗p < 10−4; ∗∗∗p < 10−3; ∗∗p < 10−2; -p > 0.05; Fisher exact test with UAS-rasV12 (green bars). Error bars indicate 95% confidence interval.
(E) Evolution of the area covered by WT cells (one circle = one region surrounded by several clones). Bars indicate averages; t test with UAS-rasV12 (green).
(F) Average rasV12 cell apical area (three first cells layers of the clones) at 0 and 700 min. Mann-Whitney tests between time points; ∗∗∗∗p < 10−4 from 4, 4, and 3 nota. Error bars indicate SEM. Upon activation of MyoII, cells are already smaller at t0 because transcription was activated 8 hr before.