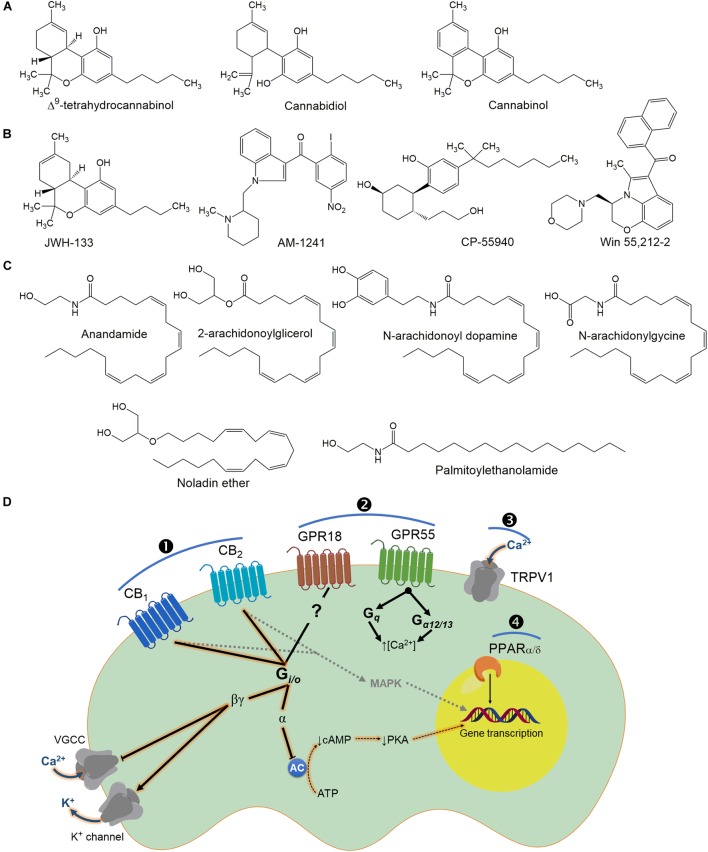
FIGURE 2.
Chemical structures of some plant (A), synthetic cannabinoids (B) and endocannabinoids (C) that bind to cannabinoid receptors (D). It is interesting to note that cannabinoids could activate intracellular pathways by direct activation of its receptors (\protect❶ and ❷) or modulate other family receptors (\protect❸ and ❹), which contribute to the biological effect of these molecules (particularly for the endocannabinoids). In general terms, classic cannabinoid receptors (CB1 and CB2) are GPCRs, which are canonically coupled to Gi/o proteins. Consequently, under CB1/2 receptors: (i) a decrease of adenylyl cyclase (AC) activity; (ii) an inactivation of Ca2+ channels; and (iii) activation of inwardly rectifying K+ channels are achieved. These are signal transduction systems associated with inhibition of neurotransmitter release. The inhibition of AC occurs via activation of Gαi-mediated signaling whereas Gαo-activation results in inhibition of voltage-dependent Ca2+ channels (VDCCs) through the release of associated βγ subunits (apparently CB2 receptors are ineffective, compared with CB1, for shifting ionic currents via βγ subunits). In addition to PKA inhibition, CB1/2 receptor signaling also leads to the downstream activation of MAPK which can regulate nuclear transcription factors and consequently expression of several genes. Note that GPR18 seems to be coupled to Gi/o proteins, whereas GPR55 has been associated with an increase of intracellular Ca2+ via Gα12/13. In the case of TRPV1 channels (a non-selective cation channel for Ca2+, Mg2+, and Na+ ions), it is well-known that agonist can be used rationally for the treatment of pain considering that this channel under constant activation desensitizes the nociceptive neuron. Finally, although not fully investigated, cannabinoid compounds could also activate PPARα/δ, which are involved in pain modulation and transmission.