Fig. 1.
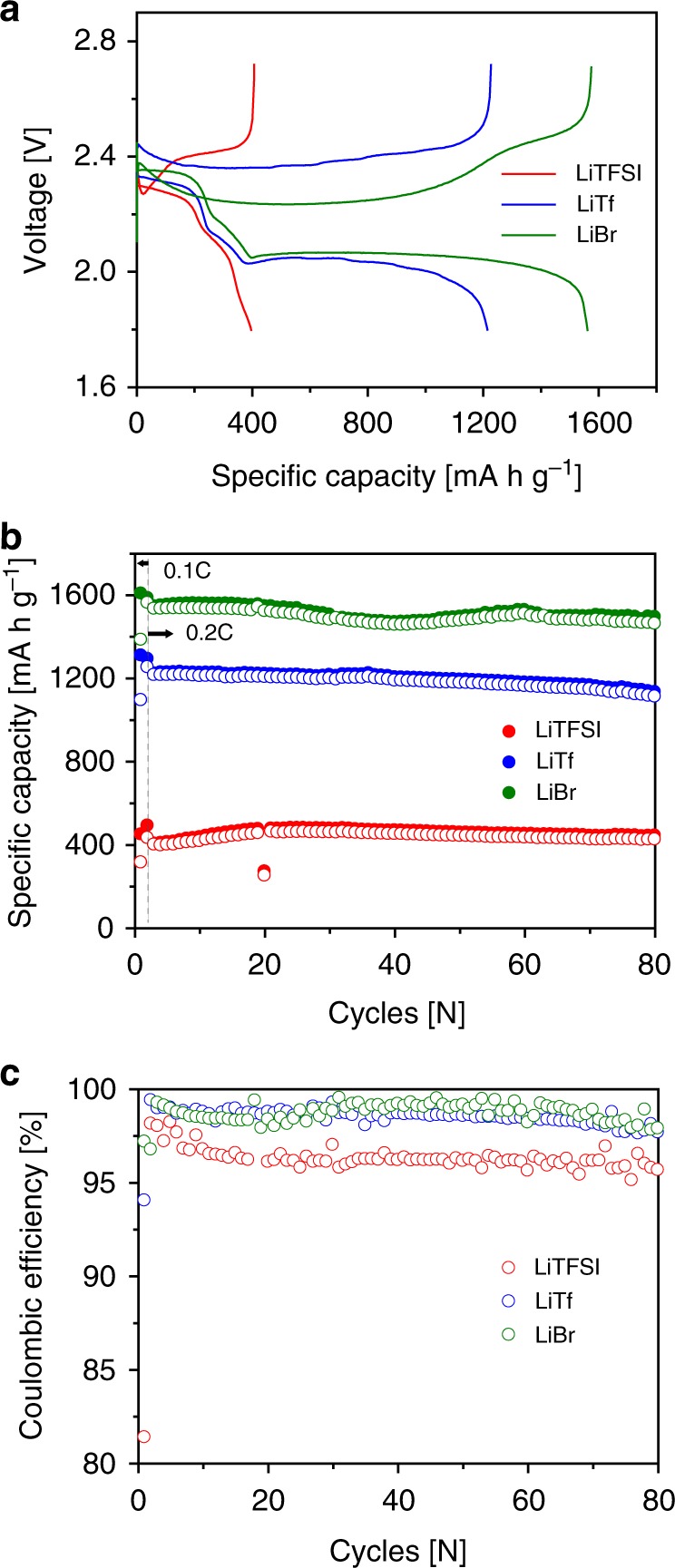
Electrochemical performances of the lithium–sulfur cells with varying anions. a Charge and discharge curves of the first 0.2 C cycle, b comparison of the charge (closed circle) and discharge (open circle) capacities, and c Coulombic efficiency data for 80 charge/discharge cycles at 0.2 C. The electrolytes consist of 0.2 M lithium polysulfide (LiPS, Li2S8 based) with 1 M Li salts LiX, X = bistriflimide (TFSI−), triflate (Tf−), or bromide (Br−) / 0.2 M lithium nitrate (LiNO3) / 1,3-dioxolane (DOL):1,2-dimethoxyethane (DME) (1:1)
