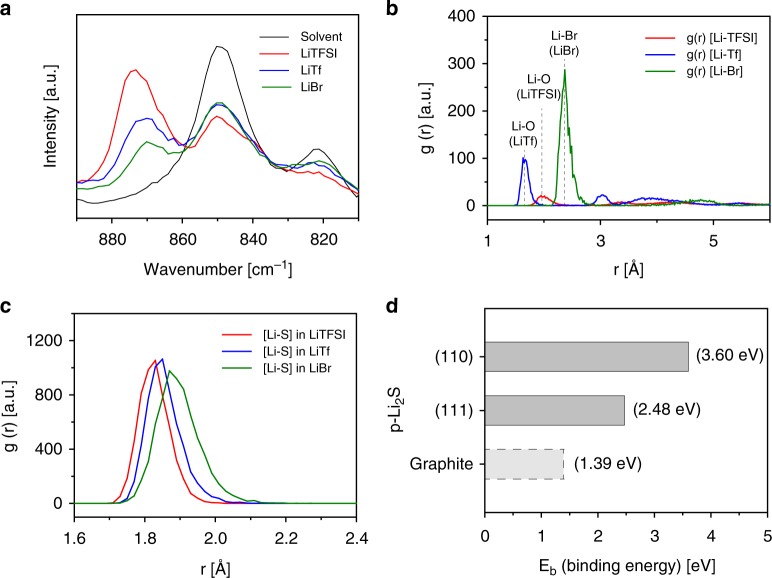
Fig. 6.
The effect of electrolyte anions on lithium sulfide solubility. a Raman spectra of 1 M LiX, X = bistriflimide (TFSI−), triflate (Tf−), or bromide (Br−), supporting salts in 1,3-dioxolane (DOL):1,2-dimethoxyethane (DME) (1:1) solutions with respect to the pure solvent mixture (black). b Radial distribution functions (how density (g(r)) varies as a function of radial distance (r)) of X− anions from lithium ion (Li+) under the 1 M LiX in DOL:DME solutions. c Radial distribution functions of lithium cation–sulfide anion (Li+–S2−) in different salt environments. d Summary of the binding energy of newly generated Li2S on different surfaces (graphitic carbon surface vs. Li2S precipitates (p-Li2S)) based on the first-principle calculations