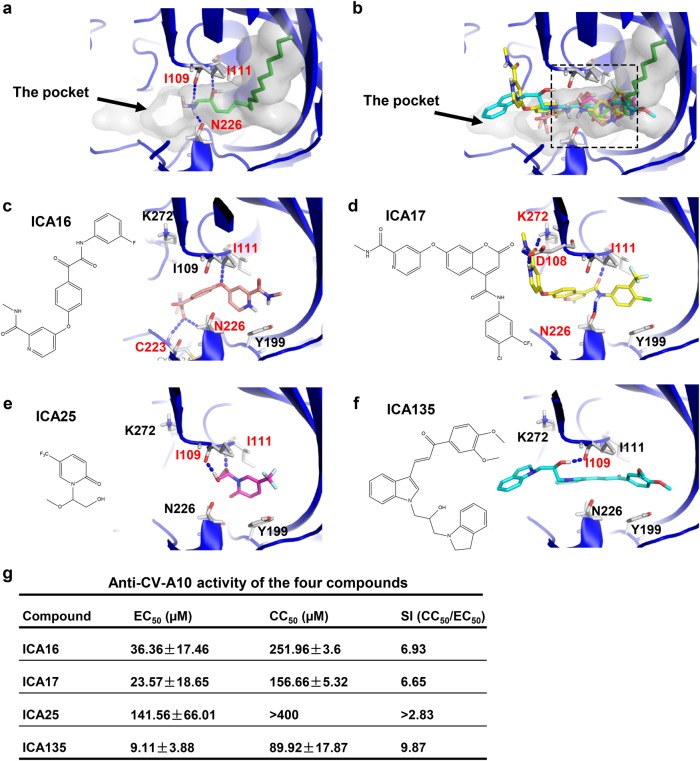
Fig. 5. Binding modes of SPH and the four pocket-binding compounds and the anti-CV-A10 activity measurement.
a The pocket factor of CV-A10, SPH, occupies part of the pocket (shown as transparent surface) and forms hydrogen bonds with I109, I111, and N226 of VP1 in CV-A10. Hydrogen bonds are shown as blue dashed lines. b Predicted binding positions of the four compounds in VP1 pocket of CV-A10 mature virion. The pocket (transparent surface) and the pocket factor are also shown to illustrate the relative locations of the four compounds. The four compounds are shown as sticks and colored by elements, with the carbon atoms of ICA16, ICA17, ICA25, and ICA135 colored in salmon, yellow, magenta, and cyan, respectively. The overlapping region inside the pocket among the CV-A10 pocket factor and the four compounds is indicated by the dashed rectangle. c–f Binding modes of ICA16 (c), ICA17 (d), ICA25 (e), and ICA135 (f) to the VP1 pocket of CV-A10 mature virion predicted by docking analysis (using Glide 6.9 in its SP mode). CV-A10 is shown in blue cartoon, and the compounds in stick. Potential hydrogen bonds formed between the pocket and the compounds are shown as blue dashed lines, and the residues involved in the hydrogen bond formation are labeled in red. g List of EC50, CC50, and SI (CC50/EC50) values of the four compounds against CV-A10 (S0148b). 50 µl of serially diluted compounds was mixed with 50 µl of CV-A10 virus containing 100TCID50. The mixtures were incubated for 1 h and then added to 2 × 104 RD cells, followed by incubation at 37 °C for 48 h. Then, culture supernatants were collected and analyzed for virus titers by plaque assays. Each experiment was performed at least three times