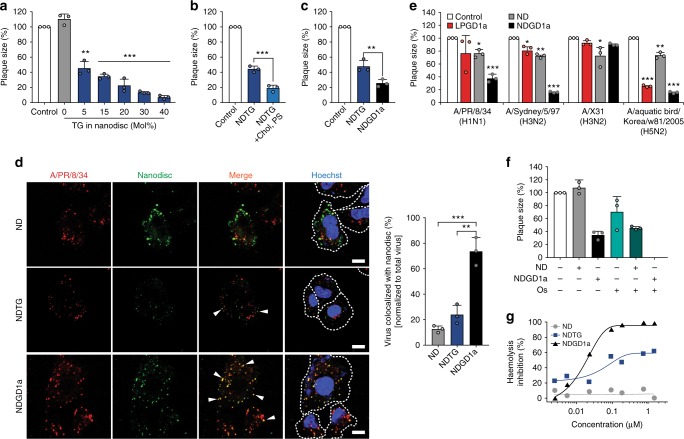
Fig. 3.
Optimisation of nano-perforator to improve anti-influenza activity. a–c Anti-influenza efficacies of nanodiscs with different formulations determined by a plaque reduction assay. Nanodiscs were prepared with various amounts of total gangliosides (a), with cholesterol and negatively-charged lipids (b), and with ganglioside GD1a (c). Nanodisc concentrations were 0.25 μM for (a) and 0.1 μM for (b, c). d Immunofluorescent images showing intracellular influenza virus–nanodisc colocalization in a receptor-dependent manner. A549 cells were incubated with SP-DiOC18-labelled influenza virus A/PR/8/34 (red) pre-mixed with Rhod-PE-labelled nanodiscs (green) at 37 °C and fixed at 2 h post-infection. Cell membranes and nuclei were stained with WGA-AF647 (white dashed line) and Hoechst (blue), respectively. Representative orange colocalization spots are indicated by white arrows. Histogram of virus–nanodisc colocalization is shown on the right. Scale bars, 10 μm. e Antiviral effects of NDGD1a (0.1 µM) against infections of multiple influenza virus strains compared with liposome containing GD1a (LPGD1a). f Combination effect of nanodiscs with oseltamivir (Os) on influenza virus H1N1 infectivity. Histogram of antiviral activity of co-treatment of 0.3 µM Os and 0.1 µM nanodiscs against A/PR/8/34 (H1N1). g Haemolysis inhibition of chicken red blood cells by nanodiscs. Data are expressed as the mean ± SD of three independent experiments (a–f). Asterisks indicate statistical significance determined by Student’s t-test (*P < 0.05; **P < 0.01; ***P < 0.001) (a–e)