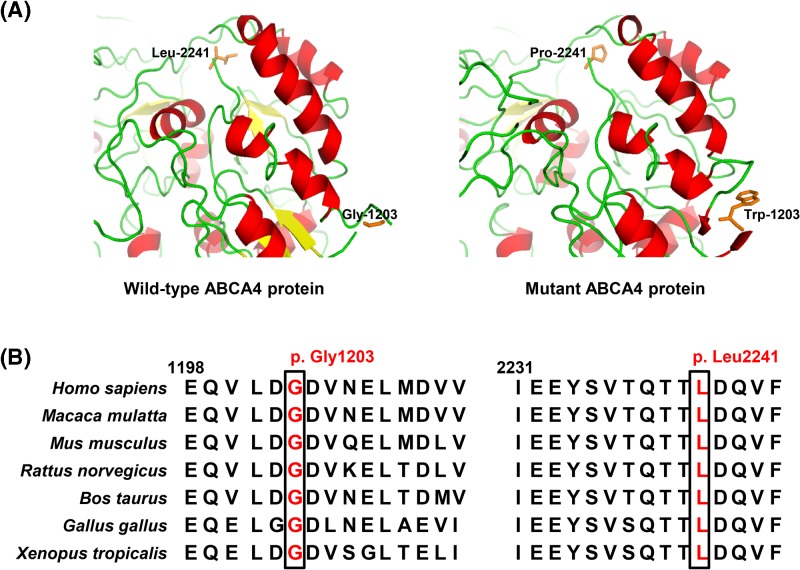
Figure 3. Structure prediction and conservation analysis of wild-type and mutant ABCA4 proteins.
(A) Predicted structures of wild-type and mutant ABCA4 proteins showed that the changes in glycine to tryptophan at position 1203 and leucine to proline at position 2241 caused conformational alteration. The crystal structure of the ABCA1 protein (PDB ID: 5XJY, 51.97% sequence identity) was used as template. (B) Multiple sequence alignment in ABCA4 proteins from human to tropical clawed frog revealed that the glycine and leucine at mutant sites were both located within highly conserved regions, suggesting that these amino acids are crucial for normal protein function.