-
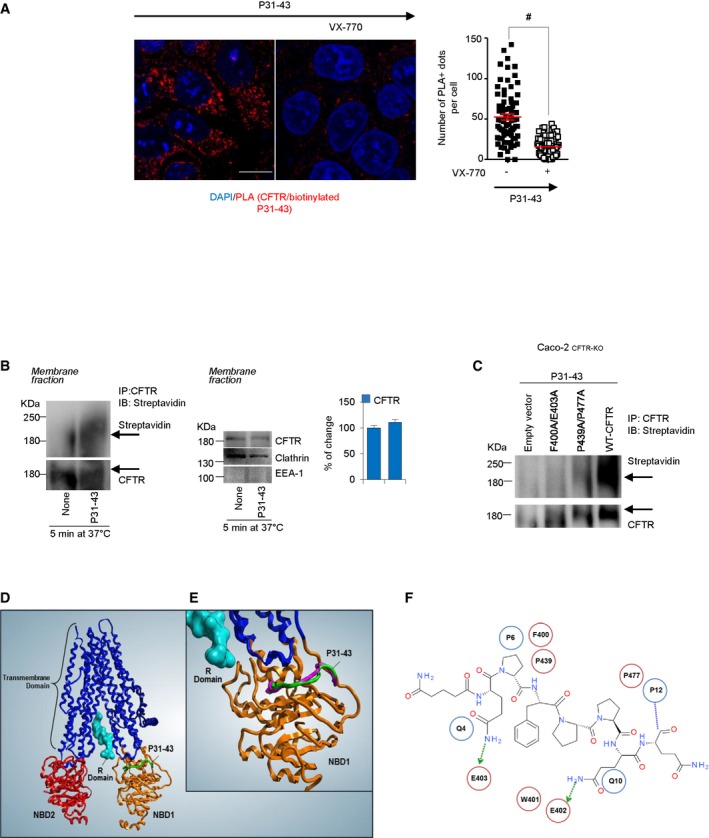
A
Caco‐2 cells challenged with P31–43 peptide in the presence or absence of pretreatment of VX‐770. Confocal analysis of endogenous CFTR interaction with P31–43 by proximity ligation assay (PLA). Scale bar, 10 μm. Quantifications were obtained from five randomly selected fields per condition, with each containing ˜ 20–25 cells. Data are presented as means ± SEM, #
P < 0.05 relative to VX‐770 + P31–43; two‐tailed unpaired Student's t‐test.
-
B
Caco‐2 cells challenged with biotinylated P31–43 for 5 min at 37°C. Immunoprecipitation of purified protein from membrane fractions in non‐reducing and non‐denaturing conditions of CFTR protein and immunoblot with streptavidin‐HRP or CFTR antibody (left). Immunoblot of purified protein from membrane fractions with anti‐CFTR, anti‐clathrin, and anti‐EEA‐1 antibodies. EEA‐1 and clathrin were used as controls of purification of clathrin‐positive membrane fraction vesicles (middle). Densitometric analysis of protein levels (right). Mean ± SD of three independent experiments.
-
C
Caco‐2CFTR‐KO cells were transfected with pcDNA3.1_F400A/E403A‐CFTR, pcDNA3.1_P439A/P477A‐CFTR, or pcDNA3.b WT‐CFTR plasmids (as positive control) or empty vector (as negative control). After 24 h, the cells were challenged with P31–43. Immunoprecipitation in non‐reducing and non‐denaturing conditions of CFTR protein and immunoblot with streptavidin‐HRP or CFTR antibody (n = 3 independent experiments).
-
D
Protein–protein docking and molecular dynamics of P31–43 (green) bound to CFTR.
-
E
Comparison between the docking poses of P31–43 against CFTR structure (green) and against NBD1 alone (violet).
-
F
2D interaction map, between P31–43 peptide retrieved from the molecular docking procedure (PDB: 2BBO). Most important amino acids are detailed (blue circle for P31–43, red circle for NBD1).
Data information: The blots are representative of one experiment for group of treatment.