Figure 2. Rolling between the two constitutive ABD dimers boosts receptor activity.
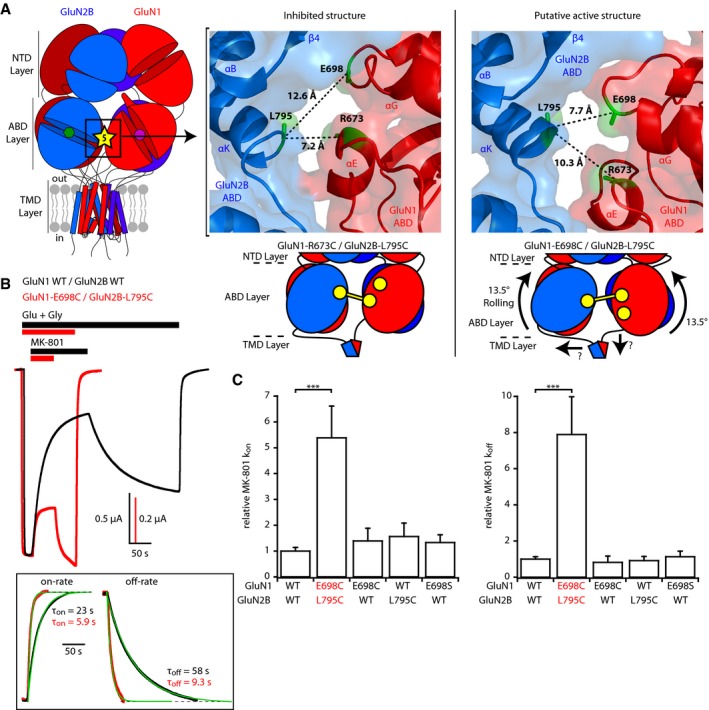
- Left, localization of site 5 at the interface between the two constitutive ABD dimers. Right top, crystal structures of the inhibited (PDB 5IOV; Zhu et al, 2016) and presumably active (5FXG; Tajima et al, 2016) states illustrating the differences in distances between GluN2B‐L795 and GluN1‐E698 or GluN1‐R673 at interface 5. Those three residues are colored green, while GluN1 and GluN2B subunits are colored red and blue, respectively. Right bottom, schematic representation of the GluN1‐R673C/GluN2B‐L795C disulfide cross‐link capturing an inhibited state of the receptor and of the GluN1‐E698C/GluN2B‐L795C disulfide cross‐link trapping the rolling motion.
- Assessment of receptor channel activity using MK‐801 inhibition kinetics. Representative current traces from oocytes expressing either wild‐type (WT) or GluN1‐E698C/GluN2B‐L795C mutant receptors in response to 10 nM MK‐801 during agonist application. Responses were scaled to the current amplitude obtained before MK‐801 application. Inset, mono‐exponential fits of MK‐801 wash‐in and wash‐out. Note the strikingly faster kinetics in mutant receptors, both at the onset and at the offset of MK‐801.
- Relative MK‐801 inhibition on‐ and off‐rate constants (k on and k off). All values were normalized to the value obtained for WT GluN1/GluN2B receptors. Mean and n values are given in Appendix Table S2. ***P < 0.001, one‐way ANOVA on ranks followed by Bonferroni‐corrected Dunn's test. Error bars, SD.
