Figure EV1. Ubiquitination of BAK by Parkin occurs in SH‐SY5Y and MEF cells on the conserved Lys113 in the hydrophobic surface groove (relates to Figs 1 and 2).
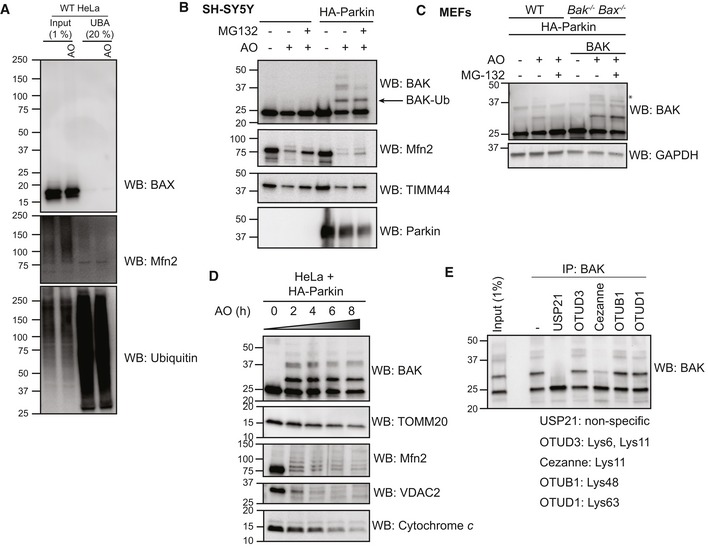
- UBA pull‐down of ubiquitinated proteins following antimycin A and oligomycin (AO) treatment of wild‐type (WT) HeLa cells for 3 h. Representative immunoblot from two independent experiments.
- Endogenous BAK is ubiquitinated in neuroblastoma cells upon mitochondrial damage. SH‐SY5Y cells ectopically expressing Parkin were treated with antimycin A and oligomycin (AO) for 2 h. Representative immunoblots of three independent experiments.
- Endogenous BAK is ubiquitinated in MEFs upon mitochondrial damage. Wild‐type MEFs (WT) or Bak −/− Bax −/− MEFs expressing hBAK and ectopically expressing HA‐Parkin were treated with antimycin A and oligomycin (AO) with or without MG132 for 2 h. Lysates were immunoblotted as described. Note that endogenous mouse BAK was ubiquitinated and that ubiquitinated forms of mouse and human BAK (*) were reduced with MG132. Representative immunoblots of three independent experiments.
- Timecourse of mitophagy induction. HeLa cells expressing HA‐Parkin were treated with antimycin A and oligomycin (AO) for the indicated times prior to immunoblotting of membrane fractions as described. Representative immunoblot from two independent experiments.
- BAK −/− BAX −/− HeLa cells expressing BAK and HA‐Parkin were treated with antimycin A and oligomycin (AO) for 2 h prior to immunoprecipitation of BAK. Immunoprecipitates were incubated with the indicated ubiquitin linkage‐specific DUB prior to analysis of BAK by immunoblotting. Data representative of three independent experiments.
Source data are available online for this figure.
