Figure 2. Parkin ubiquitinates BAK on a conserved lysine in the hydrophobic groove.
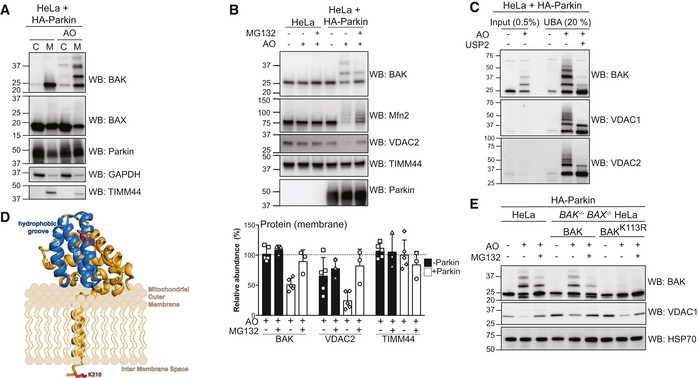
- Subcellular fractionation of cytosol and membrane fractions of HeLa cells expressing HA‐Parkin following 2 h of antimycin A and oligomycin (AO) treatment. Representative immunoblot of three independent experiments.
- Immunoblotting of whole cell lysates following treatment with antimycin A and oligomycin (AO) for 3 h with MG132 (20 μM). Graph shows densitometric analysis of non‐ubiquitinated proteins relative to untreated control from three independent experiments. Error bars represent mean ± SD.
- UBA pull‐down of ubiquitinated proteins following antimycin A and oligomycin (AO) treatment for 3 h and treatment with the non‐specific DUB USP2 for 30 min at 37°C, representative immunoblot from two independent experiments.
- Structure of human BAK (PDB 2IMS) with the hydrophobic binding groove comprising α‐helices 3–5 highlighted in blue. The ubiquitination site, K113, (red) resides in the binding groove. A modelled transmembrane anchor (α9) and the C‐terminal K210 localised to the inter‐membrane space are based on evidence that the BAK transmembrane anchor spans the mitochondrial outer membrane (Iyer et al, 2015). The orientation of the soluble portion (α‐helices 1–8) of BAK with respect to the mitochondrial outer membrane is hypothetical.
- Whole cell lysates of HA‐Parkin and BAK variant‐expressing HeLa cells following 3 h antimycin A and oligomycin (AO) treatment with 30 min pre‐treatment with MG132 (20 μM), representative immunoblots from three independent experiments.
Source data are available online for this figure.
