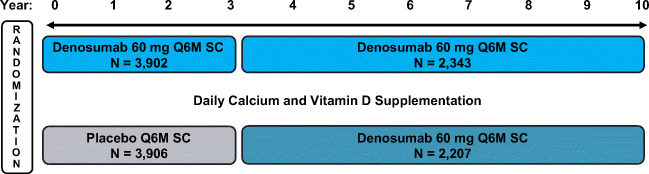
Fig. 1.
FREEDOM and FREEDOM Extension study design. This analysis included women who had received ≥ 2 doses of investigational product (placebo or denosumab) during FREEDOM or the Extension, had an osteoporotic fracture (new vertebral, including clinical vertebral, or nonvertebral) while on treatment, and continued treatment post-fracture. Q6M = every 6 months; SC = subcutaneously