Table 2.
Summarization of polymorphism of several drugs.
| Drug Substance | Polymorphism Aspects | Bioavailability Issues |
|---|---|---|
Chloramphenicol palmitate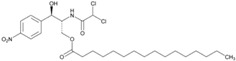
|
Chloramphenicol palmitate is a prodrug of chloramphenicol with antibiotic properties [64]. Chloramphenicol palmitate exist in three polymorphic forms: (A, B, C) [65,66], the stable form A (biologically inactive modification), the metastable form B (active modification) and unstable form C [67,68,69]. The three crystalline forms were also called α, β and γ. The α form is unstable at room temperature and gradually transforms to β on storage [70,71]. |
Form B (β) dissolves faster than Form A (α), and has a much higher solubility [72,73,74]. Low serum levels for the stable polymorph A were observed [75]. |
Oxytetracycline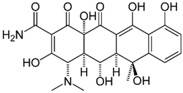
|
Oxytetracycline is a broad spectrum antibiotic. It exists in two different polymorphs [76]. |
Oxytetracycline showed differences in patients’ blood levels [77] or differences in in vitro dissolution of tablets [78] because of differences in polymorphic forms. |
Carbamazepine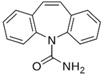
|
Carbamazepine is used in the treatment of epilepsy and trigeminal neuralgia. Different polymorphic forms were described [79,80,81,82,83,84,85,86,87,88,89,90,91]. Four anhydrous polymorphs were characterized: I, II, III, and IV, respectively identified as triclinic, trigonal, monoclinic, and monoclinic [77]. |
In spite different studies demonstrated similar pharmacokinetics in humans of anhydrous and dihydrate forms of carbamazepine [92] and no differences in bioavailability between a generic carbamazepine product and an innovator product [93], several clinical failures were reported concerning carbamazepine [94,95], in particular with generic carbamazepine tablets [96]. More recently, it was confirmed that the initial dissolution rate of carbamazepine was in the order of form III > form I > dihydrate, while the order of AUC values was form I > form III > dihydrate. This discrepancy may be attributed to the rapid transformation from form III to dihydrate in GI fluids [97]. |
Ritonavir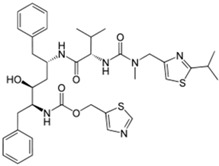
|
Ritonavir is an antiretroviral drug belonging to protease inhibitor class and used to treat HIV-1 infection. Ritonavir exhibits conformational polymorphism [98] and a total of five forms were described [60]. The forms I and II were more extensively characterized [98]. |
2 years after the launch of the first ritonavir product, several batches failed dissolution specifications because the presence of a different polymorphic form having ~50% lower intrinsic solubility of reference form [36]. |
Atorvastatin calcium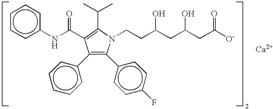
|
Atorvastatin calcium is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, with strong ability to lowering blood cholesterol. At least 60 polymorphic forms/solvates/hydrates have been patented [99,100,101]. It is not unusual to verify the presence of polymorphic impurities in the marketed atorvastatin calcium (API) with consequences on drug bioavailability and stability [102]. |
Atorvastatin is unstable and the hydroxy acid form is converted to lactone form that is 15 times less soluble than the hydroxyl acid form [103,104]. This instability of atorvastatin calcium leading to poor solubility (0.1 mg/mL) is the main cause for low bioavailability of the drug after oral administration as the absolute bioavailability of atorvastatin calcium is only 14% [105]. |
Axitinib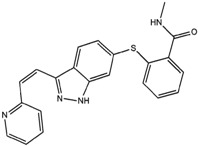
|
Axitinib is a tyrosine kinase inhibitor of endothelial growth factor interrupting tumor angiogenesis and thus, preventing the growth of cancer cells. 60 solvates, polymorphs of solvates, and five anhydrous forms were discovered [106,107,108,109]. |
The commercial formulation under trade name Inlyta® contains the stable anhydrous form [107]. |
Phanylbutazone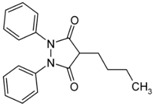
|
Phenylbutazone is a potent anti-rheumatic drug existing in different polymorphic and solvated forms [110,111,112,113]. Anhydrous forms I and II were more extensively described and form II resulted more soluble than form I. The Form III is a highly unstable form [110]. |
Anhydrous forms I and II polymorphic forms exhibited different solubilities, dissolution rates and oral absorption [110,114]. |
Rifaximin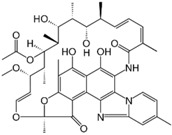
|
Rifaximin is a synthetic derivative of rifamycin, with very low gastrointestinal absorption, but still displaying a broad spectrum of antibacterial activity [115,116,117]. Rifaximin shows crystal polymorphism (poolymorphs α, β, γ, δ, ε) [118,119]. The polymorph α is the most thermodynamically stable form and the commercial one. |
In vitro studies show different dissolution and solubility rates for these polymorphs, and in vivo investigations in dogs found different pharmacokinetic patterns, with δ and γ polymorphs displaying the highest systemic bioavailability [119]. The most PK parameters were significantly higher after administration of generic rifaximin, because of the presence of both rifaximin-α and amorphous forms [120]. |
