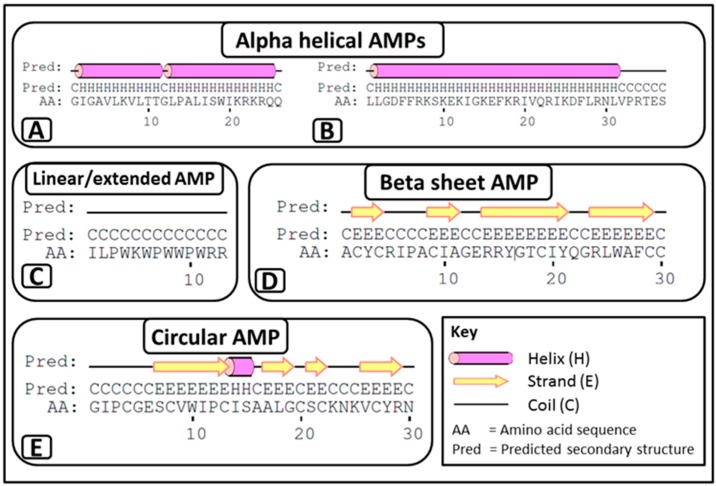
Figure 1.
Secondary structures of the three major AMP classes. Examples of α-helical AMPs include (A) melittin, two α-helixes joined by a hinge between residues 11–12; and (B) LL-37, α-helix with an unstructured segment at the C-terminal; (C) indolicidin, which can be classified as an extended coil; and (D) human neutrophil peptide 1, which is an example of a β-sheet. In addition to the three major classes, circular AMPs including (E) circulin A, consisting of both α-helix and β-sheet structures, are also found in nature. Secondary structures were generated with the PSIPRED protein prediction server [33]. Disulphide bonds are not shown.