Table 2.
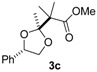
Stereoselective formation of dioxolane 3a.
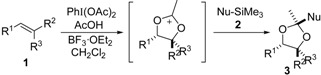
| Entry | 1 | Nu-SiMe3 | Product | Yield (%) |
|---|---|---|---|---|
| 1 b | 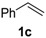 |
 |
 |
15 (1.2:1) c |
| 2 | 56 d | |||
| 3 b | 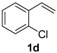 |
2a | 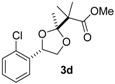 |
25 (1:1.2) c |
| 4 | 40 d | |||
| 5 |  |
2a | 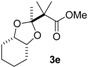 |
52 d |
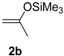
| 6 | 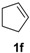 |
2a | 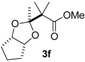 |
46 d |
| 7 | 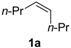 |
 |
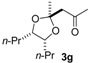 |
39 d |
| 8 | 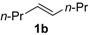 |
2b | 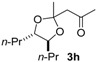 |
37 |
| 9 | 1c | 2b | 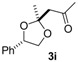 |
29 d |
| 10 | 1d | 2b | 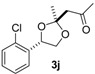 |
25 (8:1) c |
| 11 | 1a | 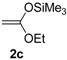 |
 |
34 d |
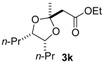
| 12 | 1b | 2c | 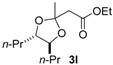 |
21 |
| 13 | 1d | 2c | 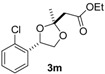 |
29 (7:1) c |
| 14 | 1b | 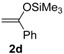 |
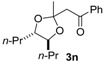 |
27 |
a Reaction was initiated at −80 °C in the presence of 1 (0.32 mmol), PhI(OAc)2 (0.40 mmol), acetic acid (0.5 mmol) and BF3·OEt2 (0.8 mmol) in CH2Cl2 (4 mL). Then, 2 (1.5 mmol) was added at −40 °C and the reaction mixture was quenched at −30 °C by the addition of water; b The reaction was quenched after warming to rt; c The value in parentheses is the diastereomeric ratio of 3 and 3′ (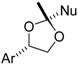 ); d Diastereomer was not detected by 1H-NMR.
); d Diastereomer was not detected by 1H-NMR.
