Table 3.
Scope of carboxylic acids a.
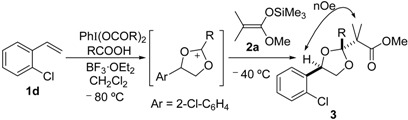
| Entry | R | Yield (%) |
|---|---|---|
| 1 | Et | 3o, 41 |
| 2 | i-Pr | 3p, 35 |
| 3 | t-Bu | b |
| 4 | Ph | 3q, 26 |
a Reaction was initiated in the presence of 1d (0.32 mmol), PhI(OCOR)2 (0.40 mmol), RCOOH (0.5 mmol) and BF3·OEt2 (0.8 mmol) in CH2Cl2 (4 mL). Then, 2a (1.5 mmol) was added at −40 °C; b No dioxolane 3 was observed and 2-hydroxy-1-arylethyl pivalate 6 was obtained in 19% yield.
