Abstract
Background: Experimental evidence supports the neuroprotective properties of lithium, with implications for the treatment and prevention of dementia and other neurodegenerative disorders. Lithium modulates critical intracellular pathways related to neurotrophic support, inflammatory response, autophagy and apoptosis. There is additional evidence indicating that lithium may also affect membrane homeostasis. Objective: To investigate the effect of lithium on cytosolic phospholipase A2 (PLA2) activity, a key player on membrane phospholipid turnover which has been found to be reduced in blood and brain tissue of patients with Alzheimer’s disease (AD). Methods: Primary cultures of cortical and hippocampal neurons were treated for 7 days with different concentrations of lithium chloride (0.02 mM, 0.2 mM and 2 mM). A radio-enzymatic assay was used to determine the total activity of PLA2 and two PLA2 subtypes: cytosolic calcium-dependent (cPLA2); and calcium-independent (iPLA2). Results: cPLA2 activity increased by 82% (0.02 mM; p = 0.05) and 26% (0.2 mM; p = 0.04) in cortical neurons and by 61% (0.2 mM; p = 0.03) and 57% (2 mM; p = 0.04) in hippocampal neurons. iPLA2 activity was increased by 7% (0.2 mM; p = 0.04) and 13% (2 mM; p = 0.05) in cortical neurons and by 141% (0.02 mM; p = 0.0198) in hippocampal neurons. Conclusion: long-term lithium treatment increases membrane phospholipid metabolism in neurons through the activation of total, c- and iPLA2. This effect is more prominent at sub-therapeutic concentrations of lithium, and the activation of distinct cytosolic PLA2 subtypes is tissue specific, i.e., iPLA2 in hippocampal neurons, and cPLA2 in cortical neurons. Because PLA2 activities are reported to be reduced in Alzheimer’s disease (AD) and bipolar disorder (BD), the present findings provide a possible mechanism by which long-term lithium treatment may be useful in the prevention of the disease.
Keywords: lithium, neuronal cell culture, iPLA2 activity, cPLA2 activity
1. Introduction
Lithium is a first-line drug for the acute and long-term treatment of bipolar disorder (BD). More recently, evidence derived from experimental models, along with data from epidemiological, neuroimaging and a few clinical studies, has reinforced the potential use of lithium for the treatment and/or prevention of dementia and related neurodegenerative conditions [1]. Lithium has been reported to play a role in neuronal homeostasis [2], stimulation of neuronal growth cones [3], up-regulation of neurotrophins brain-derived neurotrophic factor (BDNF) [4] and vascular endothelial growth factor (VEGF) [5,6], inhibition of glutamatergic excitotoxicity [7], down-regulation of autophagy [8], inhibition of β-amyloid production [9] and toxicity [3], and glycogen synthase kinase 3β-mediated Tau pathology [10].
Early studies suggested that lithium might negatively affect membrane homeostasis through the inhibition of phospholipase A2 (PLA2) [11], with relevant downstream effects on signal transduction and eicosanoid production [12]. However, a recent study from our group indicates that lithium stimulates hippocampal neurogenesis [13], an effect that apparently depends on the integrity of PLA2 function [14]. The independent assessment of distinct PLA2 subtypes, including activity and regional distribution, is probably the key to understanding the discrepancies of the enzymatic activity in Alzheimer’s disease (AD) and BD brain.
PLA2 is a superfamily of enzymes that are central to membrane phospholipid metabolism and can be divided into three main groups: secretory PLA2 (sPLA2); calcium-dependent PLA2 (cPLA2); and calcium-independent PLA2 (iPLA2) [15,16,17,18]. These enzymes generally regulate the release of lipid mediators from the cell membrane, playing important roles in signal transduction and regulation of inflammatory response [17,18,19]. Arachidonic acid (AA) is the most important and abundant free fatty acid released from membrane phospholipids through the catalytic activity of PLA2 [20,21]. Both c- and iPLA2 are highly expressed in the central nervous system, with distinct roles and regional specificities, in addition to a distinct sensitivity and pattern of response to the effect of regulatory cascades. Previous work from our group found that PLA2 activity is reduced in AD [22], and that lithium treatment reduces the risk for AD [23]. In the present study, we further develop this hypothesis by analyzing the effect of chronic lithium treatment at different working concentrations (0.02, 0.2 and 2 mM) on PLA2 activity in primary cultures of cortical and hippocampal neurons.
2. Results and Discussion
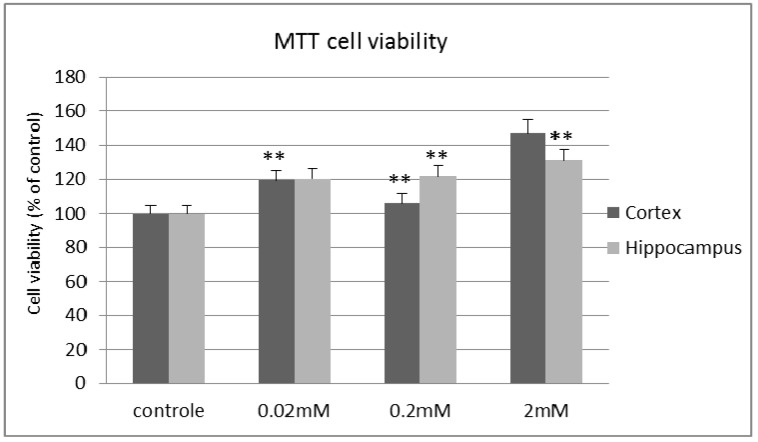
The MTT results indicate that treatment with lithium increases neuronal viability in comparison with the control cells, with a marginally significant difference (p < 0.05). Samples of cortical neurons show an increase of 19%, 6% (** p < 0.01) and 47% in the treatments of 0.02, 0.2 and 2 mM, respectively. The hippocampal cell culture samples show an increase of 20%, 21% and 31% in treatments of 0.02, 0.2 and 2 mM, respectively (Figure 1).
Figure 1.
Viabilities of primary cultures of hippocampal and cortical neurons exposed to different concentrations of lithium chloride for 7 days (n = 5, ** p < 0.01).
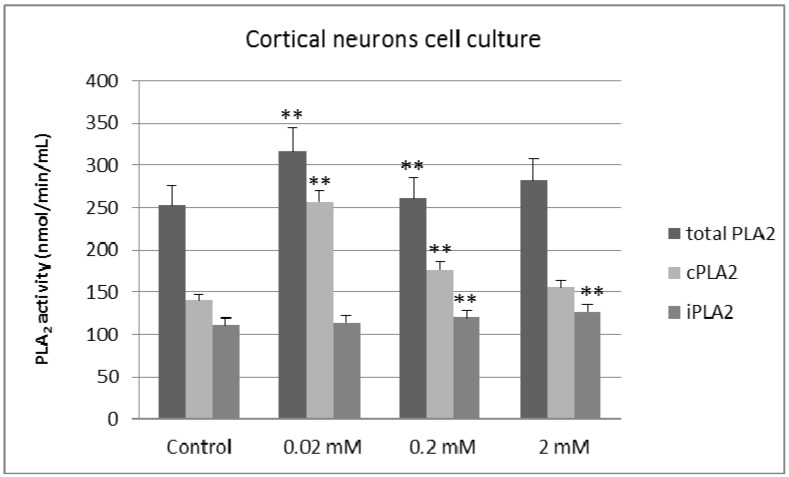
In cortical neurons, lithium treatment significantly increases total PLA2 activity by 25% (0.02 mM; p = 0.04) and 3% (0.2 mM; p = 0.035), when compared to control. cPLA2 activity increases by 82% at 0.02 mM (p = 0.05) and 26% at 0.2 mM (p = 0.04), when compared to control (Figure 2). Additionally, we found a 7% increase in iPLA2 activity at 0.2 mM (p = 0.04) and a 13% increase at 2 mM (p = 0.05) (Figure 2).
Figure 2.
Effects of different concentrations of lithium chloride on the activities of total PLA2, cPLA2 and iPLA2 in cultured cortical neurons (n = 5, ** p < 0.02).
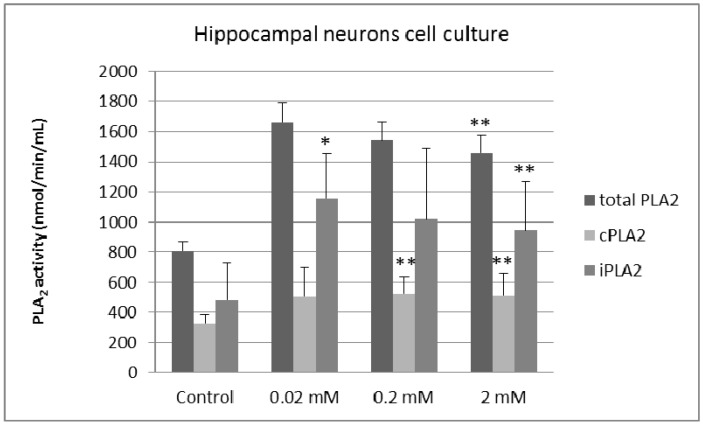
In hippocampal neurons lithium treatment significantly increased the activity of total PLA2 by 80% (2 mM; p = 0.04); cPLA2 by 61% (0.2 mM; p = 0.03) and 57% (2 mM; p = 0.04) and iPLA2 by 141% (0.02 mM; p = 0.0198), 112% (0.2 mM; p = 0.2) and 97% (2 mM; p = 0.3) (Figure 3).
Figure 3.
Effects of different concentrations of lithium chloride on the activities of total PLA2, cPLA2 and iPLA2 in cultured hippocampal neurons (n = 5, * p < 0.05, ** p < 0.02).
Our results show that long-term lithium treatment increases both cPLA2 and iPLA2 activity in primary cultures of cortical and hippocampal neurons at therapeutic and sub-therapeutic concentrations of lithium chloride. This effect was more prominent at micromolar concentrations, with tissue-specific differences in the magnitude of the effect: treatment with 0.02 mM lithium yielded a potent activation of iPLA2 in primary cultures of hippocampal neurons, with a 114% increase in enzymatic activity, whereas the same treatment in cortical neurons resulted in a predominant effect on cPLA2 (82% increase).
At first sight, the present findings seem to be in disagreement with the results from studies conducted in the late 1990’s, which suggest that lithium might actually inhibit PLA2 [11,12]. In the study by Chang and Jones [11] the effect of lithium on total PLA2 activity was determined in rat brain homogenates, whilst Chang et al. [12] did not assess directly PLA2 activity, but made rather an indirect assumption of PLA2 inhibition by showing, in vivo, that chronic treatment of adult rats with therapeutic doses of lithium decreased the turnover of arachidonate within the total brain contents of phospholipids [7]. The methodological differences between these studies and ours are probably key to the interpretation of the current findings. First, we used a different experimental model (i.e., primary neuronal cultures) to test the hypothesis. Second, we explored this effect using a wider therapeutic window of lithium, i.e., ranging from sub-therapeutic (micromolar) to therapeutic (milimolar) concentrations, which proved to have distinct effects on the target enzymes. Third, we determined the activity of PLA2 subtypes using a validated method that specifically reads c- and iPLA2 [24]. Finally, we determined PLA2 activity in primary cultures predominantly composed by hippocampal or cortical neurons; that is to say, we did not use a total brain model, in which the determination of PLA2 activity represents the sum of neuronal and glial PLA2, bearing in mind that the enzyme is more constitutively expressed and active in glial cell than in neurons.
Therefore, our data indicate that lithium positively regulates the activity of these two cytosolic forms of PLA2. Such an effect may be relevant to the understanding of the trophic effects of lithium, since both c- and iPLA2 are implicated in processes related to neurodevelopment and neuroprotection [25]. In the developing human brain, iPLA2 is mainly expressed in proliferative zones [26], which have been shown to be sensitive to the neurotrophic effect of lithium [21] including its ability to induce neurogenesis [13].
The reactivity of hippocampal iPLA2 to the effect of lithium probably relates to the neurobiological functions of the enzyme in this important cerebral structure. The hippocampus is the brain structure where iPLA2 displays its highest documented enzymatic activity [25]. In addition to a well-accepted role in signal transduction through the release of AA and other lipid mediators [27], regulating neurogenesis [26]. Evidence suggests that hippocampal iPLA2 is also implicated in the long-term potentiation (LTP) of excitatory synaptic transmission [28,29] and subsequent mechanisms of memory formation [26,28]. Early studies from Clement et al. [30] showed that AA was released through PLA2 catalytic activity at early stages of LTP induction in membranes prepared from the dentate gyrus. Drapeau and collaborators [31] also proposed that PLA2 is involved in hippocampal LTP by increasing the production of AA, which acts as a trophic retrograde synaptic signal to increase transmitter release at glutamatergic synapses [32]. The hypothesis that PLA2 facilitates transmitter release in LTP is further supported by the fact that iPLA2 participates in membrane fusion, which is a process required for exocytosis [20,33]. Previous findings from our group indicate that the specific inhibition of c- and iPLA2 impair neurite outgrowth and decrease the viability of cultured cortical and hippocampal neurons [34]. Accordingly, the inhibition of iPLA2 in the rat hippocampus also impairs acquisition of short- and long-term memory [35]. Therefore, one must consider the possibility that the activation of iPLA2 in the hippocampus may add to the myriad of neurobiological properties of lithium [36], particularly on the preservation of homeostatic mechanisms related to neuronal response to injury and memory formation [37,38,39].
The stimulatory effect of lithium on cPLA2 and iPLA2 is particularly interesting in the light of the involvement of PLA2 in the pathophysiology of AD. Abnormalities in PLA2 have been consistently described in AD patients, showing reduction of enzymatic activity both in the brain regions such as frontal and parietal cortex [40], hippocampus [41] and, peripherally, in platelets of patients with dementia and mild cognitive impairment (MCI) [39,40]. Recent findings from our group indicates that decreased iPLA2 activity predicts the risk of conversion from MCI to dementia within the MCI-AD continuum, and decreased cPLA2 predicts incident MCI in former cognitively unimpaired elders [37]. Interestingly, these abnormalities seem to respond to treatment with the antidementia drug donepezil, restoring homeostatic levels of enzymatic activity [42]. Therefore, the present findings suggest that lithium treatment may also modify a biological abnormality that is found in patients with, or at risk of AD.
3. Experimental Section
3.1. Establishment and Treatment of Primary Neuronal Cultures
Pregnant Wistar rats were sacrificed by cervical dislocation at gestational day 18 (E18), and the respective embryos were obtained by laparotomy. Whole embryonic brains were isolated and kept immersed in Hank’s balanced salt solution (HBSS; Gibco, Grand Island, NY, USA). Multiple fragments of cortical and hippocampal tissues were obtained by microdissection, followed by trypsinization (chemical dissociation) and mechanical dissociation. Single-cell suspensions were counted and re-suspended in Neurobasal medium containing B-27 supplement (Gibco), 2 mM glutamine, penicillin (100 I.U.), streptomycin (100 mg/mL), and 5% fetal calf serum (all Gibco). Cells were plated onto poly-d-lysine coated Petri dishes at a density of 1 × 107 cells per culture plate, and incubated for up to 10 days at 37 °C and 5% CO2. On day 4 after in vitro plated, hippocampal and cortical neurons were incubated for 7 days (37 °C, 5% CO2), with different concentrations of lithium (0.02 mM, 0.2 mM and 2 mM). Neuronal viability was microscopically ascertained prior to experimentation. All procedures involving laboratory animals were approved by the Ethics Committee and the Animal Care Committee of the University of São Paulo School of Medicine, in the city of São Paulo, Brazil, and were conducted in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (ISBN 0-309-05377-3, NIH publication No. 86-23, revised 1985; National Research Council 2011).
3.2. Assessment of Cell Viability
Cell viability was quantitatively assessed by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method, which estimates the percentage of living cells in a given substrate compared to controls [43]. Treatments were replicated at least 5 times with identical experimental conditions. Briefly, cells were plated in the same concentration by surface in a 96 well plate and followed the same treatments described above, after the last day (10 day in culture) of treatment, 50 μL of MTT solution (5 mg/mL in PBS) was added to each well (1 × 105 cells/mL) and the plates were incubated for 3 h, 37 °C and CO2 5%. Then, 500 μL of 10% SDS in 0.01 N HCl was added. After overnight incubation, the absorbance was measured by spectrophotometry at 570 nm.
3.3. Determination of PLA2 Activity
To determine PLA2 activity we used L-α-1 palmitoyl-2-arachidonyl-phosphatidylcholine (Perkin Elmer Life Science, New England Nuclear, Boston, MA, USA) labeled in position 2 with [114C] AA (PC-AA-[114C]) as enzyme substrate. The assay mixture contained 50 μL of 1.0 M Tris-HCl buffer pH 7.5, 200 μL of culture cell homogenates (1 mg of protein homogenate), 150 μL of PC-AA-(114C) (0.12 μCi). For cPLA2 measurement we used an optimal concentration of CaCl2 (30 μM) and the inhibitor bromoenol-lactone (BEL) to inactivate iPLA2 activity (500 μM). Total PLA2 was measured with CaCl2 5 mM and vehicle (DMSO). The solution was incubated for 30 min at 37 °C and the reaction was stopped by the addition of 700 μL isopropanol-hydrochloric acid. The 14C-labeled AA released by the cleavage of PC-AA-[114C] by PLA2 was extracted with n-heptane, followed by adsorption of the unbroken phospholipids and the lysophospholipids on 60 mg of silica. The radioactivity of free 14C-labeled AA was measured in a Tri Carb Liquid Scintillation counter (Tri-Carb 2100TR: Packard, Meriden, CT, USA). The results were given in CPM (counts per minute) and converted to picomols per milligram of protein per minute using the equation: PLA2 activity = CPM × F/A × E × 2.22 × B. The blank counts were subtracted from each sample count (where: CPM = counts per minute; F = adjustment factor for protein concentration; A = specific activity of radioactive substrate in mCi/mmol; B = incubation time in minutes; E = equipment efficiency) [24]. iPLA2 activity was inferred by calculating the difference between total PLA2 and cPLA2 [29].
3.4. Statistical Analysis
All experiments were conducted in quintuplicates, yielding mean values for PLA2 subtypes (total, c- and i-PLA2) in each treatment condition (vehicle or lithium chloride 0.02 mM, 0.2 mM and 2 mM) and model (cortical or hippocampal neurons). Independent sample Student’s t-tests were carried out to test for the statistical significance of the difference between mean values of each treatment condition compared to the respective controls. Statistical significance was set at p < 0.05. Analyses were performed using the software package SPSS-18 (SPSS Inc., Chicago, IL, USA).
4. Conclusions
We provide evidence that long-term lithium treatment activates both forms of cytosolic PLA2-cPLA2 and iPLA2- in primary cultures of cortical and hippocampal neurons. These effects were observed at therapeutic and sub-therapeutic concentrations of lithium chloride, with a more prominent effect at the micromolar range, but definitely without a dose-response pattern. We found that the effect of lithium on these two subtypes of PLA2 depends on the brain area from which the primary neurons derive, i.e., iPLA2 being more sensitive to the effect of lithium in hippocampal neurons, and cPLA2 in cortical neurons. Such differences are probably related to the distinct physiological roles and sensitivity to regulatory mechanisms of c- and iPLA2 within the brain. The present findings may be relevant to the understanding of the neurobiological mechanisms of lithium related to neurotrophic response and neuroprotection.
Acknowledgments
The present study was financially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant no. 09/52825-8, 2011/19892-3 ) and by the Brazilian Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Office for the Advancement of Higher Education). Laboratory of Neuroscience, LIM-27, receives financial support from the Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS, Alzira Denise Hertzog da Silva Benevolent Association (ABADHS) and Idenildo Ferreira from University of Edinburgh for English revisions.
Author Contributions
V.J. De-Paula conceived, designed and performed the experiments, analyzed the data, wrote the paper. M.P.F. Carvalho designed and performed the experiments. E.L. Schaeffer designed and performed the experiments and analyzed the data. D.S. Kerr analyzed the data, wrote the paper. L.L. Talib designed and performed the experiments. W.F. Gattaz analyzed the data, wrote the paper. O.V. Forlenza conceived and designed the experiments, analyzed the data, wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: All samples were prepared according to the protocols described and are not available. All reagents are commercially available.
References
- 1.Young A.H. More good news about the magic ion: Lithium may prevent dementia. Br. J. Psychiatry. 2011;198:336–337. doi: 10.1192/bjp.bp.110.082875. [DOI] [PubMed] [Google Scholar]
- 2.Chiu C.T., Chuang D.M. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol. Ther. 2010;128:281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaltiel G., Dalton E.C., Belmaker R.H., Harwood A.J., Agam G. Specificity of mood stabilizer action on neuronal growth cones. Bipolar Disord. 2007;9:281–289. doi: 10.1111/j.1399-5618.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- 4.Walz J.C., Frey B.N., Andreazza A.C., Ceresér K.M., Cacilhas A.A., Valvassori S.S., Quevedo J., Kapczinski F. Effects of lithium and valproate on serum and hippocampal neurotrophin-3 levels in an animal model of mania. J. Psychiatr. Res. 2008;42:416–421. doi: 10.1016/j.jpsychires.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda S., Liang M.H., Marinova Z., Yahyavi A., Chuang D.M. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol. Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 6.Guo S., Arai K., Stins M.F., Chuang D.M., Lo E.H. Lithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytes. Stroke. 2009;40:652–655. doi: 10.1161/STROKEAHA.108.524504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nonaka S., Hough C.J., Chuang D.M. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-d-aspartate receptor-mediated calcium influx. Proc. Natl. Acad. Sci. USA. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkar S., Floto R.A., Berger Z., Imarisio S., Cordenier A., Pasco M., Cook L.J., Rubinsztein D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phiel C.J., Wilson C.A., Lee V.M.Y., Klein P.S. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 10.Lovestone S., Davis D.R., Webster M.T., Kaech S., Brion J.P., Matus A., Anderton B.H. Lithium reduces tau phosphorylation: Effects in living cells and in neurons at therapeutic concentrations. Biol. Psychiatry. 1999;45:995–1003. doi: 10.1016/S0006-3223(98)00183-8. [DOI] [PubMed] [Google Scholar]
- 11.Chang M.C., Jones C.R. Chronic lithium treatment decreases brain phospholipase A2 activity. Neurochem. Res. 1998;23:887–892. doi: 10.1023/A:1022415113421. [DOI] [PubMed] [Google Scholar]
- 12.Chang M.C., Grange E., Rabin O., Bell J.M., Allen D.D., Rapoport S.I. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci. Lett. 1996;220:171–174. doi: 10.1016/S0304-3940(96)13264-X. [DOI] [PubMed] [Google Scholar]
- 13.Schaeffer E.L., Cerulli F.G., Souza H.O.X., Catanozi S., Gattaz W.F. Synergistic and additive effects of enriched environment and lithium on the generation of new cells in adult mouse hippocampus. J. Neural Transm. 2014;121:695–706. doi: 10.1007/s00702-014-1175-5. [DOI] [PubMed] [Google Scholar]
- 14.Schaeffer E.L., Gattaz W.F. Chronic inhibition of brain phospholipase A2 in adult rats impairs the survival of newborn mature neurons in the hippocampus. J. Neural Transm. 2015;122:619–628. doi: 10.1007/s00702-014-1305-0. [DOI] [PubMed] [Google Scholar]
- 15.Murakami M., Naraba H., Tanioka T., Semmyo N., Nakatani Y., Kojima F., Ikeda T., Fueki M., Ueno A., Oh-ishi S., et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 16.Balsinde J., Winstead M.V., Dennis E.A. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/S0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 17.Sun G.Y., Xu J., Jensen M.D., Simonyi A. Phospholipase A2 in the central nervous system: Implications for neurodegenerative diseases. J. Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Farooqui A.A., Horrocks L.A. Brain phospholipases A2: A perspective on the history. Prostaglandins Leukot. Essent. Fatty Acids. 2004;71:161–169. doi: 10.1016/j.plefa.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Brown W.J., Chambers K., Doody A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: Mediators of membrane shape and function. Traffic. 2003;4:214–221. doi: 10.1034/j.1600-0854.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 20.Rapoport S.I. In vivo fatty acid incorporation into brain phosholipids in relation to plasma availability, signal transduction and membrane remodeling. J. Mol. Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. Discussion 279–284. [DOI] [PubMed] [Google Scholar]
- 21.Quiroz J.A., Machado-Vieira R., Zarate C.A., Manji H.K. Novel insights into lithium’s mechanism of action: Neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62:50–60. doi: 10.1159/000314310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattaz W.F., Maras A., Cairns N.J., Levy R., Förstl H. Decreased phospholipase A2 activity in Alzheimer brains. Biol. Psychiatry. 1995;37:13–17. doi: 10.1016/0006-3223(94)00123-K. [DOI] [PubMed] [Google Scholar]
- 23.Nunes P.V., Forlenza O.V., Gattaz W.F. Lithium and risk for Alzheimer’s disease in elderly patients with bipolar disorder. Br. J. Psychiatry. 2007;190:359–360. doi: 10.1192/bjp.bp.106.029868. [DOI] [PubMed] [Google Scholar]
- 24.Talib L.L., Diniz B.S., Zainaghi I.A., Forlenza O.V., Gattaz W.F. A radioenzymatic assay to identify three groups of phospholipase A2 in platelets. Prostaglandins Leukot. Essent. Fatty Acids. 2012;86:149–153. doi: 10.1016/j.plefa.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Forlenza O.V., Schaeffer E.L., Gattaz W.F. Phospholipase A2 activity in rat embryonic brain and in primary cultures of cortical neurons. J. Neural Transm. 2002;109:623–631. doi: 10.1007/s007020200051. [DOI] [PubMed] [Google Scholar]
- 26.Polster B., Crosier M., Lindsay S., Hayflick S. Expression of PLA2G6 in human fetal development: Implications for infantile neuroaxonal dystrophy. Brain Res. Bull. 2010;83:374–379. doi: 10.1016/j.brainresbull.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazan N.G., Zorumski C.F., Clark G.D. The activation of phospholipase A2 and release of arachidonic acid and other lipid mediators at the synapse: The role of platelet-activating factor. J. Lipid Mediat. 1993;6:421–427. [PubMed] [Google Scholar]
- 28.Wolf M.J., Izumi Y., Zorumski C.F., Gross R.W. Long-term potentiation requires activation of calcium-independent phospholipase A2. FEBS Lett. 1995;377:358–362. doi: 10.1016/0014-5793(95)01371-7. [DOI] [PubMed] [Google Scholar]
- 29.Lucas K.K., Dennis E.A. Distinguishing phospholipase A2 types in biological samples by employing group-specific assays in the presence of inhibitors. Prostaglandins Other Lipid Mediat. 2005;77:235–248. doi: 10.1016/j.prostaglandins.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Clements M.P., Bliss T.V., Lynch M.A. Increase in arachidonic acid concentration in a postsynaptic membrane fraction following the induction of long-term potentiation in the dentate gyrus. Neuroscience. 1991;45:379–389. doi: 10.1016/0306-4522(91)90235-G. [DOI] [PubMed] [Google Scholar]
- 31.Drapeau C., Pellerin L., Wolfe L.S., Avoli M. Long-term changes of synaptic transmission induced by arachidonic acid in the CA1 subfield of the rat hippocampus. Neurosci. Lett. 1990;115:286–292. doi: 10.1016/0304-3940(90)90470-T. [DOI] [PubMed] [Google Scholar]
- 32.Williams J.H., Errington M.L., Lynch M.A., Bliss T.V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989;341:739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]
- 33.Takuma T., Ichida T. Role of Ca2+-independent phospholipase A2 in exocytosis of amylase from parotid acinar cells. J. Biochem. 1997;121:1018–1024. doi: 10.1093/oxfordjournals.jbchem.a021688. [DOI] [PubMed] [Google Scholar]
- 34.Fujita S., Ikegaya Y., Nishikawa M., Nishiyama N., Matsuki N. Docosahexaenoic acid improves long-term potentiation attenuated by phospholipase A2 inhibitor in rat hippocampal slices. Br. J. Pharmacol. 2001;132:1417–1422. doi: 10.1038/sj.bjp.0703970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendes C.T., Gattaz W.F., Schaeffer E.L., Forlenza O.V. Modulation of phospholipase A2 activity in primary cultures of rat cortical neurons. J. Neural Transm. 2005;112:1297–1308. doi: 10.1007/s00702-004-0271-3. [DOI] [PubMed] [Google Scholar]
- 36.Camins A., Verdaguer E., Junyent F., Yeste-Velasco M., Pelegrí C., Vilaplana J., Pallás M. Potential mechanisms involved in the prevention of neurodegenerative diseases by lithium. CNS Neurosci. Ther. 2009;15:333–344. doi: 10.1111/j.1755-5949.2009.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forlenza O.V., de Paula V.J., MacHado-Vieira R., Diniz B.S., Gattaz W.F. Does lithium prevent Alzheimer’s disease? Drugs Aging. 2012;29:335–342. doi: 10.2165/11599180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez F., Lucas J.J., Avila J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimers Dis. 2013;33:S141–S144. doi: 10.3233/JAD-2012-129025. [DOI] [PubMed] [Google Scholar]
- 39.Forlenza O.V., De-Paula V.J.R., Diniz B.S.O. Neuroprotective effects of lithium: Implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem. Neurosci. 2014;5:443–450. doi: 10.1021/cn5000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gattaz W.F., Cairns N.J., Levy R., Förstl H., Braus D.F., Maras A. Decreased phospholipase A2 activity in the brain and in platelets of patients with Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 1996;246:129–131. doi: 10.1007/BF02189113. [DOI] [PubMed] [Google Scholar]
- 41.Ross B.M., Moszczynska A., Erlich J., Kish S.J. Phospholipid-metabolizing enzymes in Alzheimer’s disease: Increased lysophospholipid acyltransferase activity and decreased phospholipase A2 activity. J. Neurochem. 1998;70:786–793. doi: 10.1046/j.1471-4159.1998.70020786.x. [DOI] [PubMed] [Google Scholar]
- 42.Talib L.L., Hototian S.R., Joaquim H.P.G., Forlenza O.V., Gattaz W.F. Increased iPLA2 activity and levels of phosphorylated GSK3B in platelets are associated with donepezil treatment in Alzheimer’s disease patients. Eur. Arch. Psychiatry Clin. Neurosci. 2015 doi: 10.1007/s00406-015-0600-6. [DOI] [PubMed] [Google Scholar]
- 43.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]