Table 1.
Catalyst screening and optimization of the three-component reactions a.
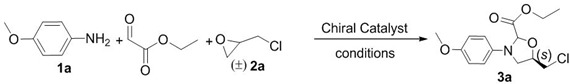
| Entry | Solvent | Catal. | T (°C) | Yield (%) b | d.r. c | ee (%) d |
|---|---|---|---|---|---|---|
| 1 | CH2Cl2 | 4a | 18 | 5 | 2:1 | 10 |
| 2 | CH2Cl2 | 4b | 18 | 13 | 3:1 | 15 |
| 3 | CH2Cl2 | 4b | −10 | 13 | 2:1 | 30 |
| 4 e | CH2Cl2 | 4c/Ti(IV) | −40 | 13 | 3:1 | 41 |
| 5 e | PhCH3 | 4c/Ti(IV) | −40 | 15 | 4:1 | 60 |
| 6 e | PhCH3 | 4c/Ti(IV) | −40 | 50 | 4:1 | 61 |
| 7 f | PhCH3 | 4c/Ti(IV) | −40 | 53 | 11:1 | 72 |
| 8 f | PhCH3 | 4d/Ti(IV) | −40 | 36 | 11:1 | 72 |
| 9 f | PhCH3 | 4c/Ti(IV) | −55 | 30 | 12:1 | 73 |
| 10 f | PhCH3 | 4c/Ti(IV) | −70 | 15 | 12:1 | 73 |
a 1a (1.1 mmol) and ethyl glyoxalate (1.0 mmol) were stirred for 1 h in 1.5 mL solvent, then 2a (0.2 mmol) and catalyst (0.1 mmol) were added, and the system was stirred for 4 days; b Yields of isolated products; c,d were determined by HPLC on a chiral column; e Catalysts were prepared by Ti(IV) and 4c ligand in a 1:1 molar ratio and TFA was added into the reactions as a catalyst; f Catalysts were prepared by Ti(IV) and 4c, 4d ligands in a 1:2 molar ratio and TFA was added into the reactions as a catalyst.
