Table 2.
Three-component reaction of anilines, ethyl glyoxalate, and epoxides a.
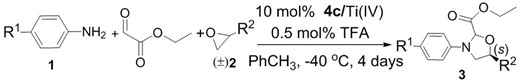
| Entry | 3 | R1 | R2 | Yield (%) b | d.r. c | ee (%) d |
|---|---|---|---|---|---|---|
| 1 | 3a | CH3O | CH2Cl | 52 | 12:1 | 43 |
| 2 | 3b | Cl | CH2Cl | 50 | 10:1 | 69 |
| 3 | 3c | CH3CH2O | CH2Cl | 47 | 10:1 | 39 |
| 4 | 3d | CH3O | CH2OCH(CH3)2 | 56 | 4:1 | 43 |
| 5 | 3e | CH3O | CH2O(CH2)3CH3 | 46 | 1.5:1 | 71 |
| 6 | 3f | CH3CH2O | CH2O(CH2)3CH3 | 53 | 3:1 | 34 |
| 7 | 3g | CH3CH2O | CH2OCH(CH3)2 | 53 | 3:1 | 72 |
| 8 | 3h | Cl | CH2O(CH2)3CH3 | 48 | 3:1 | 69 |
| 9 | 3i | Cl | CH2OCH(CH3)2 | 54 | 4:1 | 90 |
| 10 | 3j | CH3O | Ph | 42 | 1.7:1 | 84.6 |
| 11 | 3k | NO2 | CH2Cl | trace | ― | ― |
a Reactions were carried out under optimum conditions; b Yields of isolated products; cdetermined by 1H-NMR; d determined by HPLC on a chiral column.
