Figure 3. Polycystin 1 downregulates Ca2+ influx and NHA2 expression.
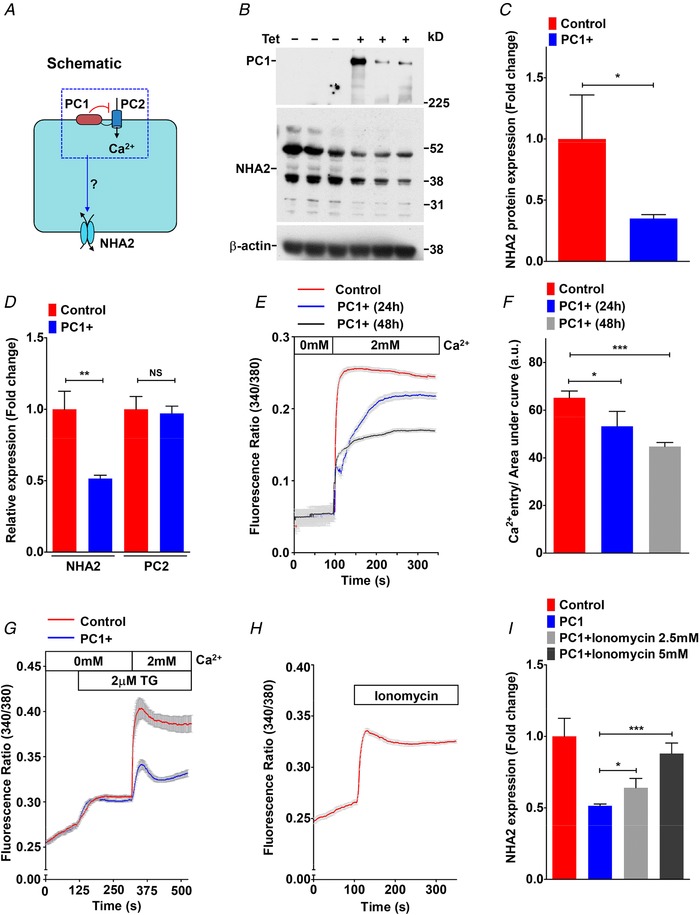
A, hypothesis for PC1‐mediated inhibition of Ca2+ influx through PC2 and NHA2 expression in MDCK cells. B, western blotting of MDCK cell lysates in the absence or presence of tetracycline for 17 h to induce PC1 expression, in biological triplicates, probed with antibody to PC1 (top), NHA2 (middle) and β‐actin (bottom). C, densitometric quantification of western blot shown in B. NHA2 protein expression was normalized to actin controls and was significantly down‐regulated by ∼2.9‐fold (n = 3; Student's t test, * P < 0.05) in MDCK cells with PC1 induction. D, quantitative PCR (qPCR) showing significant down‐regulation of NHA2 mRNA (n = 3; Student's t test, ** P < 0.01) and no change in PC2 mRNA (n = 3; Student's t test, NS P > 0.05) with PC1 induction. E and F, representative Fura‐2 fluorescence ratio traces (E) and quantitation (F) showing significant and proportionate reduction in store‐independent calcium entry (SICE) upon induction of PC1 for 24 h (n = 3; Student's t test, * P < 0.05) and 48 h (n = 3; Student's t test, *** P < 0.001) in MDCK cells. G, representative Fura‐2 fluorescence ratio traces showing reduction in store‐operated calcium entry (SOCE) following thapsigargin‐mediated release of store Ca2+ in MDCK cells with PC1 induction for 48 h. H, representative Fura‐2 fluorescence ratio traces showing efficacy of Ca2+ ionophore ionomycin to enhance cytosolic Ca2+ levels. I, qPCR showing significant and dose‐dependent increase in NHA2 expression with ionomycin treatment to levels similar to MDCK cells without PC1 induction. Error bars are S.D.
