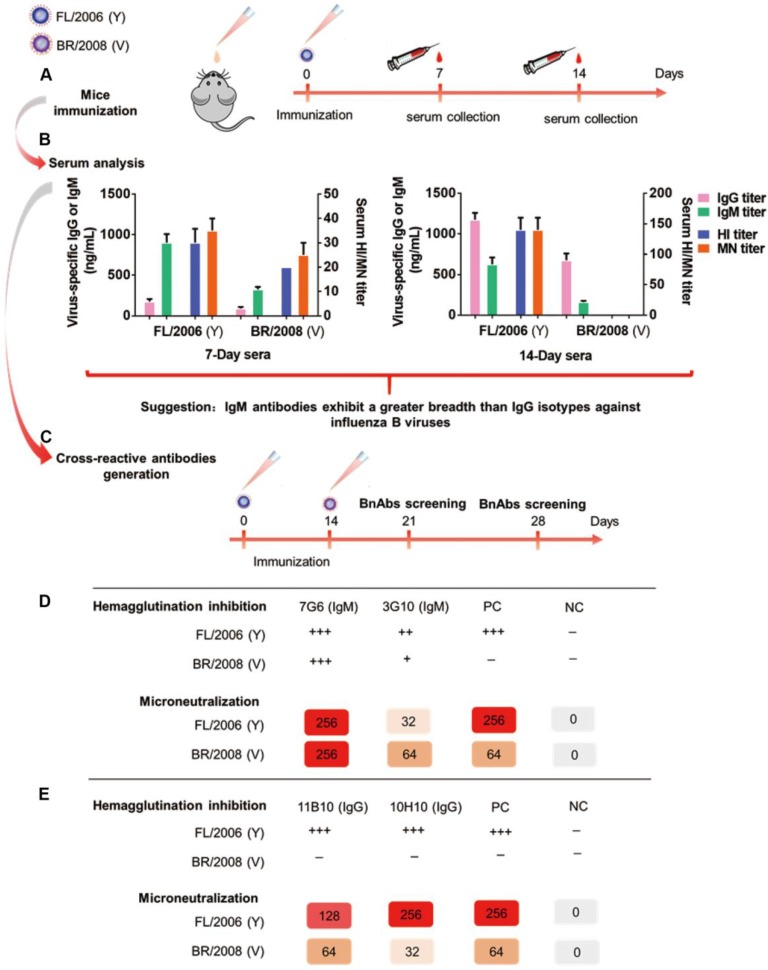
Figure 1.
Schematic showing the generation of bnAbs. (A) Serum collection from mice (n=6) immunized intranasally with the Yamagata lineage virus strain FL/2006 (B/Florida/4/2006, blue cartoon particle) at 1×104 TCID50 per mouse. The mice were immunized at day 0 and sera were collected 7 and 14 days after each immunization. (B) Characterization of 7-day and 14-day anti-influenza sera following intranasal immunization with the Yamagata lineage strain of influenza B virus. Shown are data for serum total IgG titers, serum total IgM titers, serum HI titers and serum MN titers of FL/2006-immunized sera against two representative influenza B viruses, FL/2006 and BR/2008 (B/Brisbane/60/2008, Victoria lineage), analyzed in parallel. Recombinant HA proteins of FL/2006 and BR/2008 were used as ELISA plate-coating antigens. IgG and IgM titers were determined with quantitative ELISA and are expressed in ng/mL. Bars represent averages and standard errors. (C) A schematic depicting the generation of cross-reactive antibodies. Mice (n=8) were sequentially infected intranasally with FL/2006 and BR/2008 at 1×104 TCID50 per mouse. 21 or 28 days after the first immunization, spleen cells were collected from infected mice and fused with mouse myeloma Sp2/0 cells. (D) Hybridomas from the 21-day fusion groups were screened for production of cross-lineage mAbs specific to influenza B virus using HI and MN assays against FL/2006 and BR/2008 viruses. (E) Hybridomas from the 28-day fusion groups were screened for production of cross-lineage mAbs specific to influenza B virus using HI and MN assays against FL/2006 and BR/2008 viruses. +++, strong reactivity; ++, moderate reactivity; +, weak reactivity; -, no reactivity. PC: positive control, antibody CR8033-like. NC: negative control, antibody CR9114-like.