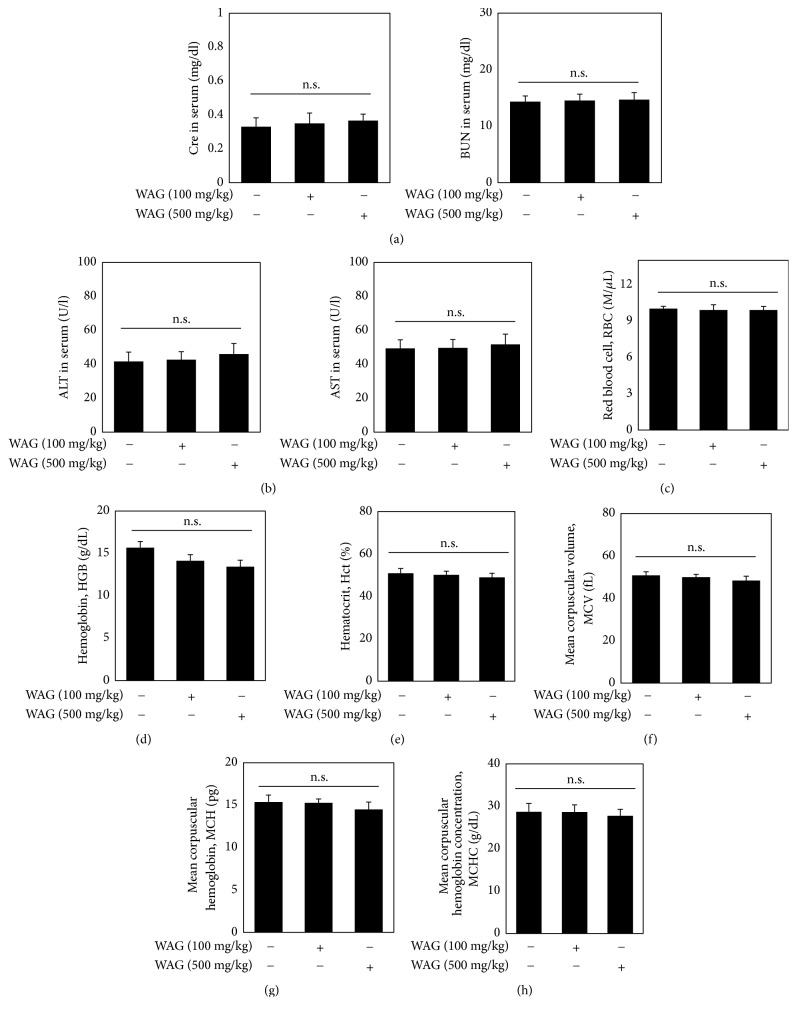
Figure 6.
Toxicity and hematological evaluation of WAG administration on toxicity markers of kidneys, liver, and blood. (a-b) Mice were separated into three groups (NOR; n=10, 100 mg/kg WAG; n=10, 500 mg.kg WAG; n=10). Mice were administered with WAG (100 or 500 mg/kg). BUN, Cre, AST, and ALT were analyzed by FUGI DRI-CHEM 4000i. (c-h) Hematological parameters including RBC (red blood cell), HGB (hemoglobin), Hct (hematocrit), MCV (mean corpuscular volume), MCH (mean corpuscular hemoglobin), and MCHC (mean corpuscular hemoglobin concentration) were analyzed by IDEXX ProCyte. Data represent the mean ± SEM of three independent experiments. Means with difference letters are significantly different at #p < 0.05 vs. normal group; ∗p < 0.05 vs. CIA group by one-way ANOVA with Tukey post hoc test. CIA, collagen induced arthritis; MTX, methotrexate; WAG, water extracts of Acori Graminei Rhizoma.