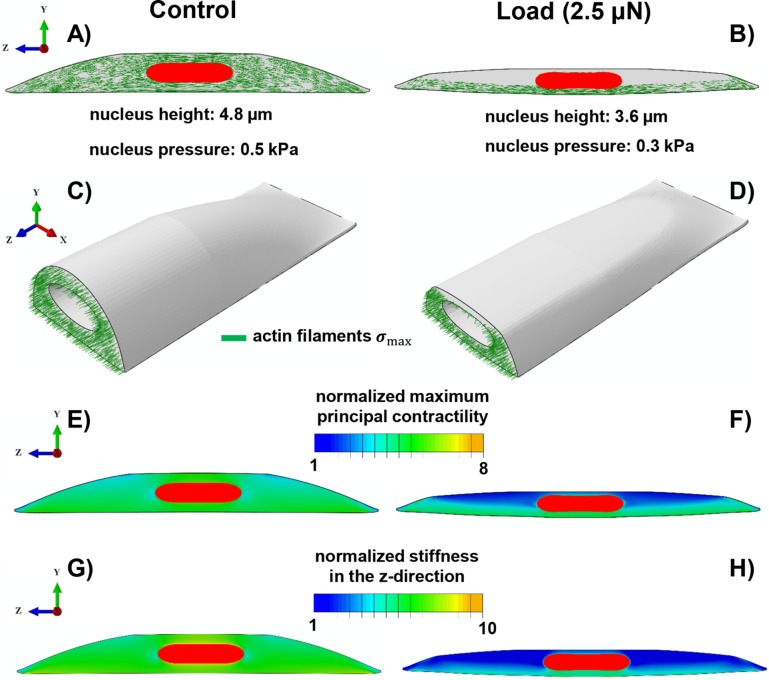
FIGURE 5:
Simulation results of the mechanochemical model depicting the role of compressive forces on apical actin and nuclear morphology. Starting with isotropic contractility (the same concentration of the phosphorylated myosin motors in all directions), our model predicts an increase in the density of the phosphorylated myosin motors (density of force dipoles) along the long axis of the cell (i.e., polarized contraction). The polarized contraction results in higher tensile stresses along the cell’s polarization direction and subsequently formation of actin filaments along the long axis of the cell (A, C) as observed in our experiments (see Figure 4A). A compressive force of 2.5 µN causes a significant reduction in the cell contractility (a decrease in the density of phosphorylated myosin motors) in the apical regions as shown in F. This reduction in the contractility leads to a decrease in the tensile stresses in the apical regions and subsequently depolymerization of apical actin filaments as shown in B and D. Our model also predicts a decrease in cell stiffness associated with the depolymerization of apical actin filaments in response to the compressive force as depicted in H when compared to G.