Abstract
Benthic—pelagic coupling plays a pivotal role in aquatic ecosystems but the effects of fishery driven interactions on its functioning has been largely overlooked. Disentangling the benthic—pelagic links including effects of mixed fisheries, however, needs sketching a whole description of ecosystem interactions using quantitative tools. A holistic food web model has been here developed in order to understand the interplay between the benthic-pelagic coupling and mixed fisheries in a Mediterranean system such as the Strait of Sicily. The reconstruction of the food web required review and integration of a vast set of local and regional biological information from bacteria to large pelagic species that were aggregated into 72 functional groups. Fisheries were described by 18 fleet segments resulting from combination of fishing gears and fishing vessel size. The input-output analysis on the food web of energy pathways allowed identifying effects of biological and fishery components. Results showed that the structure of the Strait of Sicily food web is complex. Similarly to other Mediterranean areas, the food web of the Strait of Sicily encompasses 4.5 trophic levels (TLs) with the highest TLs reached by bluefin tuna, swordfish and large hake and largely impacted by bottom trawling and large longline. Importantly, benthic-pelagic coupling is affected by direct and indirect impacts among groups of species, fleets and fleets-species through the whole trophic spectrum of the food web. Moreover, functional groups able to move on large spatial scales or life history of which is spent between shelf and slope domains play a key role in linking subsystems together and mediate interactions in the Mediterranean mixed fisheries.
Introduction
Fishing activities targeting benthic and demersal organisms are usually considered unrelated to those targeting pelagic species, and independently managed. In fact, benthic and pelagic domains have been often treated as separate subsystems. However they are not, because of physical [1,2] and biological processes [3] that couple the two domains. As a consequence, fishery driven processes on benthic domain can have a cascading impact on demersal and pelagic domains, and vice-versa. The benthic—pelagic coupling (BPC) plays a pivotal role in aquatic ecosystems by allowing nutrient cycling and energy transfer between the benthic and pelagic domains [4]. In biogeochemistry we often refer to BPC to describe the chemical relationships between nutrient availability and primary producers. Hence, previous studies on BPC have focused mainly on the rules that drive plankton dynamics and sediment biogeochemical processes [5,6]. Little attention has been paid to the role of BPC in a more complex food web especially involving higher trophic levels in concurrency with fisheries interactions.
The release of fecal pellets in the pelagic domains sinking to deeper domains, the occurrence of “marine snow”, the re-suspension of organic material sunk on the bottom [7–9] are examples of processes contributing to BPC, although even more complex and indirect linkages should be considered. Trophic interactions contribute to BPC through movement, feeding habits or behavior of organisms. For example, vertical migrations and horizontal movements of zooplankton, which depend on food availability and predator avoidance mechanisms [10,11] may allow the transport of biomasses, nutrients and energy between coastal and pelagic and between surface and deep domains. Many marine organisms move among habitats during the day [12–14] and their predation and consumption constitute a net transfer of energy between the benthic, demersal and pelagic domains thus allowing for their coupling. Furthermore, ontogenetic diet shifts associated to different habitat preference across life stages represent a net energy flow between different domains. Benthic and pelagic domains are linked by pelagic predators such as tuna and swordfish feeding also on demersal resources [15] while pelagic preys such as sardines and anchovies may feed demersal predators [16]. However, the contribution of trophic interactions to BPC, while highlighted in the literature [3,17] has rarely been quantified in a comprehensive and holistic ecosystemic view.
Fishing is among the most worrisome stressor to BPC across short temporal scales [4,18] and the progressive expansion of fisheries to deeper environments [19,20] has the potential to produce unprecedented disturbances on deep communities with detrimental consequence on ecosystems [21–25]. Indeed, fishing can severely impact on taxa relevant for BPC [26] also through mortality of organisms in their different life stages. Unwanted catches discarded at sea constitute organic matter that sinks to the sea bottom contributing to BPC. Bottom trawling also leads to structurally simpler bottom habitats and impoverished benthic communities [27,28] which may result in fewer chances to exploit energy sources from the water column and leave physical factors to play a major role in structuring BPC. In addition, fishing has been shown to affect trophic interactions, like e.g. with the removal of large predators whose effects propagate through trophic cascade [29,30]. Therefore, the direct and indirect impacts of a mixed fishery might induce important effects on ecosystem dynamics, especially in oligotrophic and semi-enclosed systems such as the Mediterranean Sea [9], and might induce a re-organization of benthic-pelagic fluxes.
The effects of fishing on the functioning of BPC in an ecosystem context, which is a crucial aspect in the Ecosystem-Based Fishery Management (EBFM) [31], has been addressed by several authors generally with the perspective of assessing the multiple effects of trawling on seabed and on traits of benthic organisms [22,32] but the explicit quantification of the contribution of fish and fisheries to BPC needs to be better investigated. In this context ecosystem modelling represents a backbone quantitative way to investigate the role of marine communities and fisheries in BPC. Food web models have been increasingly used to study the effects of fishing and other human activities on the marine ecosystem functioning according to EBFM [33–35], also in the Mediterranean Sea [36]. By integrating large data sets from different sources, such models can represent: i) trophic interactions among the wide biological communities from plankton to top predators, ii) fishing activities with their target and non-target catches, and iii) the effects of fishing and other stressors on all fluxes among functional groups in the food web, including those involved in the BPC and the trade-offs between different fisheries mediated by ecosystem response.
Based on a standard food web ECOPATH model, this paper presents a novel application to the Strait of Sicily (SoS) ecosystem specifically developed for studying the BPC in the Mediterranean Sea. The SoS hosts one of the largest Mediterranean bottom trawl fisheries in terms of fleets, landings and economical incomes [37] and, at the same time, it is characterized by relatively high productivity and biodiversity, wide bathymetric range and habitat complexity [38]. Moreover, a section of SoS fishery targets deep water shrimps by potentially threatening deeper areas [39,40] and possibly influencing BPC also at those depths. In particular, the study allows to identify direct and indirect effects among species, among fleets and between fleets and species. In this way, we used the case of the SoS in order to investigate about the complex nature of the BPC including Mediterranean mixed fisheries. The analysis of the food web model is an attempt to assess how trophic and fishery driven interactions participate directly and indirectly to the BPC in a Mediterranean marine ecosystem.
Material and methods
Study area and modelling approach
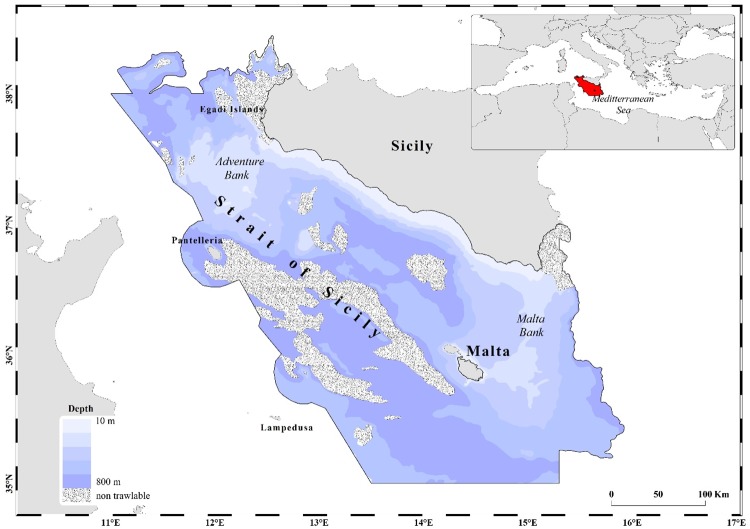
The study area of the food web model coincides with the northern side of the Strait of Sicily, which stretches off the southern Sicily coast and is characterized in its central portion by a narrow continental shelf that separates two wider portions of shelf coinciding with the Adventure Bank in the west and the Malta Bank in the east (Fig 1). The study area has a complex bottom morphology due to the presence of sedimentary and volcanic seamounts [41] that influences the hydrology in the region [42]. The shape of the slope is extremely irregular, incised by several trenches and steep areas. Sea water circulation achieves a two-layer exchange, with an inflow of the Atlantic Ionian Stream flowing eastwards (0–150 m depth) and an undercurrent composed mainly of Levantine Intermediate Water flowing in the opposite direction [43]. Persistent cyclonic vortices around the Adventure and Malta Banks produce upwelling at their center to counterbalance the divergence of surface water [44], whereas frequent wind-induced upwelling events boost primary production in coastal zones [45]. Stable environmental conditions identified around the two banks highly contribute to sustain spawning and nursery areas of commercial species [44,46–49] and hot-spots of biodiversity [50].
Fig 1. Area of the food web model (about 61,000 km2) applied to the Strait of Sicily.
The food web model is built using the Ecopath with Ecosim software (EwE v. 6.5, http://www.ecopath.org) [33]. In particular, the Ecopath module was used to integrate a massive amount of environmental, biological and fisheries information in a coherent framework and to describe yearly biomass and flows in the SoS area on the basis of a quantitative mass-balance approach that is widely detailed elsewhere (e.g., [33]) and briefly described in S1 Appendix. We have developed the food web model for the period 2004–2005 considering an area of about 61000 km2 at depths ranging between 10 and 800 m, excluding non-trawlable area represented by zones interdicted to fishing, zones deeper than 800 m and hard/rocky substrates (Fig 1). The model area has been defined on the basis of the ecological, bathymetric and fisheries-related information available for the regional area.
Biological and fisheries input data
The model development required access, review and analysis of data in the SoS for approximately 1400 taxa. Species-specific parameters and dietary data were compiled mainly from publicly available, published and unpublished information, as detailed in S1 Table. In particular, we reviewed and used experimental quantitative information on diet items from the SoS and adjacent areas for more than 200 species.
Fishery-independent biomass density estimates encompassing demersal fishes, cephalopods and other invertebrates were obtained from bottom trawl surveys carried out in spring 2004 and 2005 in the model area within the MEDITS program (Mediterranean International bottom Trawl Surveys) [51]. MEDITS followed a stratified sampling design with proportional allocation of hauls in depth strata (10–50 m, 51–100 m, 101–200 m, 201–500 m, 501–800 m), producing standardized relative biomass estimates per haul and per species (CPUE). Nevertheless, the system equations of EwE are based on absolute biomass, thus MEDITS catches by haul were processed to obtain an average absolute biomass density of each species in the slope and in the shelf portions of the SoS area. MEDITS data by haul were transformed into absolute biomass density data (of the species in the wild, kg km-2), by considering species-specific catchabilities. Since the catchability of MEDITS trawl surveys has been seldom studied and is not available for the SoS, we used specific catchability terms derived by the comparison of MEDITS data with independent stock assessments [52] and with surveys with a more efficient gear (beam trawl) from other Mediterranean areas [53] (Tyrrhenian Sea) [24] (Adriatic Sea). Such comparisons (sensu FAO [54]) provided catchability for main species that were compared with published data (e.g. [55]) whenever possible. For European pilchard Sardina pilchardus and anchovy Engraulis encrasicolus, experimental acoustic surveys carried out in the SoS were taken into account [55,56]. The mega-macrobenthic biomass data set retrieved by the MEDITS was implemented by averaging samples from surveys carried out off the northern Sicily coast as detailed in Romano et al., (2016) [57] (S1 Table). Primary productivity and biomass of phytoplankton, zooplankton, and the concentration of suspended detritus were estimated by averaging the results obtained by the COPERNICUS MedMFC products from the study area [58]. Overall, density estimates for more than 800 taxa were obtained.
For highly migratory species such as bluefin tuna Thunnus thynnus and swordfish Xiphias gladius we have used the average biomass estimates provided by stock assessments in the Mediterranean Sea [59] taking into account that both species spend a large part of their lifetime outside the SoS area. More specifically, considering the bluefin tuna migratory patterns and assuming a residing time in the modelled area of 4 months year-1 [60,61], only 30% of food consumption was considered to occur within the model area (and 70% was set as “import” fraction in the diet). In order to balance the relevant swordfish catches instead, an immigration rate of 0.025 t km-2 y-1 was considered as the minimum flow to assure mass-balance.
Fishery landings by species and gear in the SoS during 2005 were drawn from the national monitoring of commercial fleets within the European Data Collection Regulation (IREPA; www.irepa.org). Data were aggregated by 18 fleet segments resulting from a combination of fishing gears (i.e., trawlers, purse-seiners, long-liners, netters, etc.) and 3 vessel size classes based on the length overall (LOA): class 1, LOA <12m; class 2, LOA 12-24m; class 3, LOA >24m, bottom otter trawl were also distinguished into categories according to main target species (Table 1). Since the fishing activity of larger bottom trawler targeting deep water species (i.e. deep-water rose shrimp Parapenaeus longirostris and giant red shrimp Aristaeomorpha foliacea) span over a space larger than the model area [62,63], only 50% of their catches were retained inside the model area (i.e. fleet 18, Table 1) [62]. Empirical discard ratio for commercial species and invertebrates by species and by fleet was drawn from project reports on studies carried out in the SoS [63–67]. For all the other species whose data were not available, it was considered a discard ratio from nearby Mediterranean areas [68–70].
Table 1. List of the fleet segments considered by the combination of vessel size (i.e. length overall = LOA) and fishing gear.
| N° | LOA | fishing gear | fleet segment |
|---|---|---|---|
| 1 | 1 | setgill and trammel net demersal fish | 1.GNS |
| 2 | 1 | set and drifting longline | 1.LLD |
| 3 | 1 | trolling | 1.LTL |
| 4 | 1 | hand-pole cephalopods and finfish | 1.LH |
| 5 | 1 | mixed | 1.MIS |
| 6 | 1 | purse and boat seine | 1.PS |
| 7 | 2 | pots and traps demersal and small pelagic fish | 2.FPO |
| 8 | 2 | setgill and trammel net demersal fish | 2.GNS |
| 9 | 2 | set and drifting longline | 2.LLD |
| 10 | 2 | mixed | 2.MIS |
| 11 | 2 | bottom otter trawl_demersal fish | 2.OTB_D |
| 12 | 2 | bottom otter trawl_deep water species | 2.OTB_DWS |
| 13 | 2 | bottom otter trawl_mixed demersal and deep water species | 2.OTB_MDD |
| 14 | 2 | mid water otter trawl_mixed demersal and pelagic species | 2.OTM |
| 15 | 2 | pelagic pair trawl_small pelagic fish | 2.PTM |
| 16 | 2 | purse and boat seine | 2.PS |
| 17 | 3 | mid water otter trawl_mixed demersal and pelagic species | 3.OTM |
| 18 | 3 | bottom otter trawl_mixed demersal and deep water species | 3.OTB_MDDW |
LOA1 = vessel size <12m, LOA2 = 12-24m; LOA3 = >24m.
The definitions of fish functional groups (FGs) were based on a first assessment of diet similarities and life history parameters of involved species using multivariate analysis techniques in order to cluster species with similar diets, growth and mortality rate. FGs definitions were then refined on the basis of expert opinion in order to better describe ecological and fishing features of the SoS. Commercially important species were considered as single FGs, with the red mullet and the European hake represented in 4 and 5 size classes, respectively. Moreover, several FGs were split into shelf and slope components and each group labeled as benthic, demersal or pelagic (Table 2) in order to better represent the BPC. In this way, the starting list of 1400 taxa (from bacteria to large pelagic species) was reduced to 69 living FGs and 3 non-living organic matter compartments.
Table 2. Summary description of 72 biological functional groups.
| N° | Dom | Name | FG |
|---|---|---|---|
| 1 | p | Seabirds | SB |
| 2 | p | Marine mammals | MM |
| 3 | p | Sea turtles | TUR |
| 4 | p | Sword fish | XIP |
| 5 | p | Bluefin tuna | THU |
| 6 | p | Large pelagic fish | LPL |
| 7 | p | Medium pelagic fish | MPL |
| 8 | p | Other small pelagic fish | SPL |
| 9 | d | European hake<6 cm | HAK0 |
| 10 | d | European hake 6–12 cm | HAK1 |
| 11 | d | European hake 12.1–22.0 cm | HAK2 |
| 12 | d | European hake 22.1–41.0 cm | HAK3 |
| 13 | d | European hake >41.0 cm | HAK4 |
| 14 | d | Red mullet<8 cm | MUL0 |
| 15 | d | Red mullet 8–12 cm | MUL1 |
| 16 | d | Red mullet 12.1–17 cm | MUL2 |
| 17 | d | Red mullet>17 cm | MUL3 |
| 18 | d | Horse meckerel | TRA |
| 19 | d | Pandora | PAG |
| 20 | d | Demersal fish (slope) | DFS |
| 21 | d | Demersal fish crustacean feeders (shelf) | DFH |
| 22 | d | Demersal fish mixed food (shelf) | DSM |
| 23 | d | Demersal fish piscivorous (shelf) | DSP |
| 24 | d | Demersal fish rocky (shelf) | DSR |
| 25 | d | Mesopelagic fish crustacean feeders (slope) | MSC |
| 26 | d | Mesopelagic fish jelly feeders (slope) | MSG |
| 27 | d | Mesopelagic fish piscivorous (slope) | MSP |
| 28 | d | Rays and skates (shelf) | RSH |
| 29 | d | Rays and skates (slope) | RSS |
| 30 | d | Sharks (shelf) | SSH |
| 31 | d | Sharks (slope) | SSS |
| 32 | p | European anchovy | ENG |
| 33 | p | European pilchard | SAR |
| 34 | p | Epipelagic fish | EPI |
| 35 | d | Cephalopods benthic (shelf) | CEBH |
| 36 | d | Cephalopods benthic (slope) | CEBS |
| 37 | d | Cephalopods pelagic (shelf) | CEPH |
| 38 | d | Cephalopods pelagic (slope) | CEPS |
| 39 | d | Decapods natant (slope) | DNS |
| 40 | d | Decapods natant (shelf) | DNH |
| 41 | d | Decapods reptant (slope) | DRS |
| 42 | d | Decapods reptant (shelf) | DRH |
| 43 | d | Giant red shrimp | ARF |
| 44 | d | Deep water rose shrimp | PWL |
| 45 | b | Suprabenthos | SUP |
| 46 | b | macrobenthos omnivore | O |
| 47 | b | macrobenthos filter-feeder | FF |
| 48 | b | macrobenthos deposit-feeder | DF |
| 49 | b | macrobenthos carnivore | C |
| 50 | b | macrobenthos parasite | PAR |
| 51 | b | macrobenthos scavenger | SCA |
| 52 | b | macrobenthos herbivore | H |
| 53 | b | macrobenthos grazer | GRA |
| 54 | b | macrobenthos suspension-feeder | SF |
| 55 | b | macrobenthos particulate-feeder | PF |
| 56 | b | Meiobenthos | BO |
| 57 | p | Euphausiacea | EUP |
| 58 | p | Gelatinous zooplankton | ZG |
| 59 | p | Large zooplankton | ZL |
| 60 | p | Mesozooplankton | ZM |
| 61 | p | Microzooplankton | ZS |
| 62 | p | Pelagic bacteria | PB |
| 63 | b | Sediment bacteria | BB |
| 64 | p | Pico-phytoplankton | PS |
| 65 | p | Dinoflagellates | DFL |
| 66 | p | Diatom | PL |
| 67 | b | Microphytobenthos | MB |
| 68 | b | Seagrass | SG |
| 69 | b | Macroalgae | MA |
| 70 | b | Detritus Carrion | DC |
| 71 | d | Suspended Particulate Organic Matter | SPOM |
| 72 | b | Benthic Detritus | BD |
Dom = domain: p = pelagic, d = demersal, b = benthic. FG = functional group.
Biomass and catches (landings plus discards) by FG were obtained by summing over the species belonging to each group. Other input parameters (i.e., production per unit of biomass, P/B and consumption per unit biomass, Q/B estimated with empirical parameters) and diet data for each FG were obtained as a weighted average of the values for the species in that group (S1 Table), with the proportion of local species biomass within the group used as the weighting factor [71].
Analysis of interactions in the BPC context
The basic features of the food web were defined by ecological indices such as Trophic Level (TL), Primary production on Respiration (PP/R) and Primary Production on Biomass (PP/B). The Omnivory Index (OI) and the System Omnivory Index (SOI) were calculated to quantify the width of the trophic spectrum for each FG and as a measure of food web complexity and interconnection, respectively [72].
In order to evaluate the role of all food web components on the BPC we calculated fluxes and impacts among FGs, among fleets and between FGs and fleets. The fluxes are a direct output of the model and were used to determine the strength of direct interactions (i.e., amount of consumption) among FGs and across the three domains considered (i.e., benthic, demersal and pelagic). Direct fishing effects were quantified by i) the exploitation rate E (E = F/Z, where F is the annual fishing mortality and Z is total annual mortality) for each FG belonging to a certain domain, and ii) the cumulative exploitation rate (CumE) for any fleet segment. CumE was calculated (1) in order to compare exploitation across fleets and consequently to visualize which domain was more impacted.
| (1) |
The application of the input-output analysis on the web of flows i.e., the Mixed Trophic Impact analysis (MTI) [73] allowed to quantify the direct and indirect impacts among all biological FGs and fleets. The squared matrix MTI represents the impact of each FG (rows: impacting groups) on any other group of the web (columns: impacted groups). The sum of the MTI elements in the rows allows to obtain the overall cumulative impact (ɛi) produced by a component (biological group or fleet) i on the whole food web [74], while the sum by columns allows to obtain the cumulative impact suffered by a component i.
Therefore, in order to assess the impact of each FG on BPC, we calculated the portion of cumulative overall impact both positive and negative of a FG on the FGs belonging to the other domains (e.g., FG_demersal on FGs_benthic and pelagic). Finally, we explored the overall effects of fisheries across FGs and fleet segments respectively and the main cascades relevant for BPC.
Results
Model validation
Biomass and catches used in the Ecopath model resulted highly correlated (R2 = 0.75, F1,28 = 29.88, p<0.001) to those from stock assessment and other sources (Fig 2A). Some discrepancies appeared for biomass of anchovy (g) and Giant red shrimp (i) and for catches of red mullet at age class two (d). In particular, for some species such as Aristaeomorpha foliacea (i) and Engraulis encrasicolus (g) the differences in the areas to which stock assessments and Ecopath refer might explain the discrepancies in the biomasses. Higher catches of red mullet in Ecopath than in assessment models could be instead attributed to higher discards represented in the former. Ecopath mortality rates (Fig 2B) fall in the range of estimates available from stock assessments and were sufficiently correlated (R2 = 0.52, F1,30 = 90.61, p<0.001). Nevertheless, the comparison highlighted that the integration of data provided by Ecopath results in a general underestimation of fishing mortalities and overestimation of natural mortalities with respect to stock assessments. Globally, variables and parameters used in our model resulted reconciled.
Fig 2. Comparison of variables and parameters for species with detailed stock assessments.
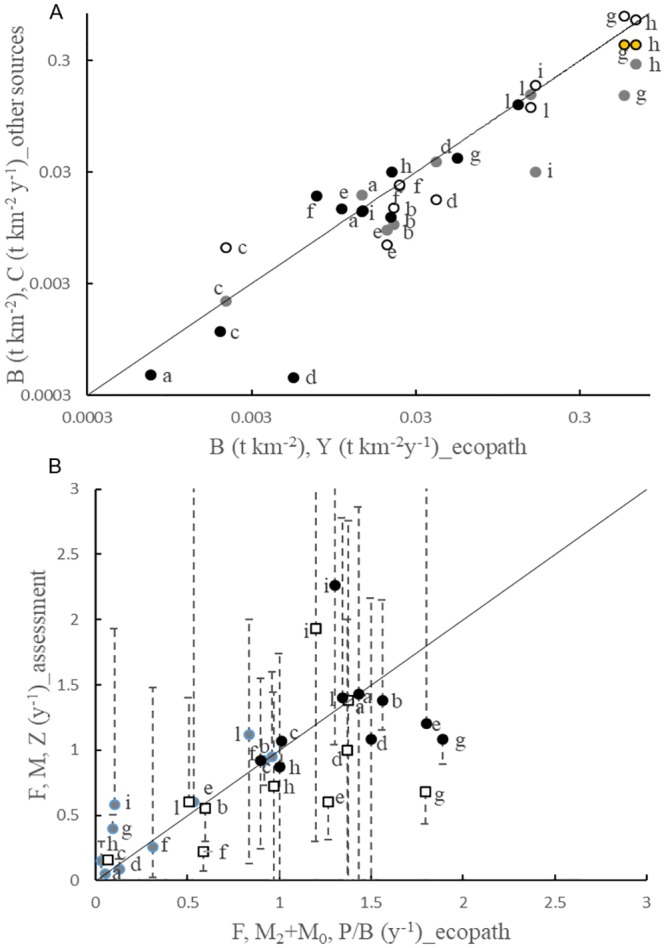
(A) Comparison of biomass and catches used in the Ecopath model (x-axis) and other sources of information (y-axis). Biomass (t km-2): gray = Ecopath vs stock assessment, white = Ecopath vs MEDITS corrected for catchability, orange = Ecopath vs acoustic-survey only for ENG (g) and SAR (h). Catches (t km-2 y-1): black = Ecopath vs stock assessment. (B) Comparison of annual mortality rates (y-1) used in the Ecopath model and stock assessments. Whiskers indicate MAX and MIN used in stock assessments for different age classes corresponding to Ecopath stanzas. Gray dot = Fishing mortality (F), white square = natural mortality (M) or sum of predation and other mortality (M2+M0), black dot = total mortality (P/B or Z). a = HAK2, b = HAK3, c = HAK4, d = MUL2, e = MUL3, f = PAG, g = ENG, h = SAR, i = ARF, l = PWL.
Structure of SoS food web and direct interactions
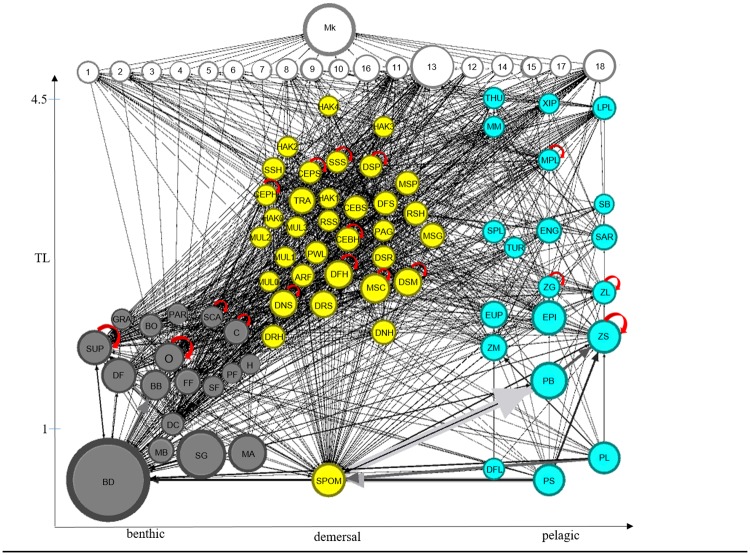
According to the model, the food web of the Strait of Sicily encompasses 4.5 trophic levels (TLs, Fig 3). The highest TLs were reached by bluefin tuna (THU = 4.55 TL), swordfish (XIP = 4.51) and large hake (HAK4 = 4.48), immediately followed by FGs large pelagic fish (LPL = 4.46) and marine mammals (MM = 4.36). The remaining FGs had TL ranging between 4.31 and 2.87 for fish, and between 2.83 and 2 for macro-benthos and bacteria. Low TL groups had generally higher biomass than those with higher TL thus determining an overall pyramid structure with vertex up. PP/R and PP/B indicated a quite mature ecosystem with a similar contribution of total production (1561 t km-2 year-1) and consumption (1599 t km-2 year-1) to the total flows in the system (total system throughput, TST). Total respiratory and detritus flows corresponded to 962 and 967 t km-2 year-1, respectively.
Fig 3. Flow diagram of the food web.
Functional groups (nodes) by trophic levels (TL, y-axis) and by benthic (gray), demersal (yellow) and pelagic (cyan) domains (x-axis). White nodes represent fishing activities and the market (Mk). Links width are proportional to flow intensity, i.e., to annual food consumption rates for FG (>5^-6 t km−2 year−1), to catches for fleets and to landings for the market. Node radius is proportional to the square root of FG biomass, total catch of fleets and total landings for the market. Gray arrows indicate higher fluxes. Red arrows are loops (cannibalism).
Globally, FGs resulted well interconnected as shown by SOI = 0.30 and by the fact that more than 50% of total consumer FGs showed a relatively high variability of feeding across trophic levels (OI>0.3).
The bulk of consumption fluxes (~95%, 1500 t km-2 years-1) was exchanged by lower “taxonomic” groups (i.e. macro-benthos, zooplankton, euphausiids and bacteria) across the benthic and pelagic domains (Fig 3). The remaining consumption flux (~ 5%) was determined by all other FGs, with epipelagic fish (EPI) and mesopelagic fish crustacean feeders (MSC) having the most relevant flows for BPC both as consumers and sources (Fig 4). Many other FGs (e.g., horse mackerel Trachurus spp., TRA and sardine, SAR), contributed to a less extent to the consumption fluxes linked to BPC (Figs 3 and 4) although they had high biomass and were predators and preys across benthic and pelagic domains as well.
Fig 4. BPC by flux (consumption) of functional groups (FG) acting as consumers (left) and as sources (right) on the 2 other domains they do not belong to.
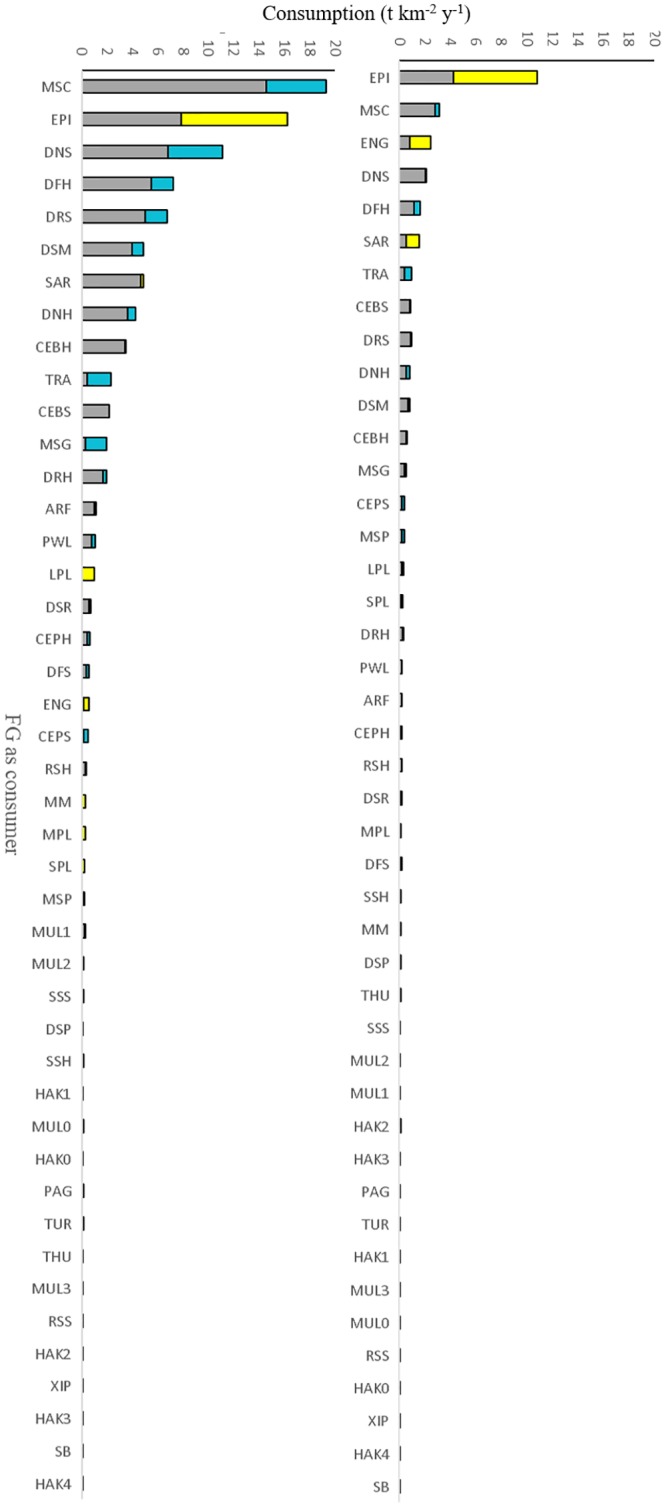
Pelagic (cyan), demersal (yellow), benthic (gray), lower trophic level groups are excluded.
The SoS fishing fleet segments, which target resources from shelf and slope, directly impact all three domains with a prevalence of impacts on the demersal and pelagic domains (Fig 5). Bottom trawlers of LOA classes 2 and 3 showed higher values of cumulative exploitation rate followed by long-liners and purse seiners of LOA class 2 (Fig 5). As a consequence, the most highly exploited groups were swordfish (XIP), large European hake (HAK3, HAK4), deep-water rose shrimp (PWL) and bluefin tuna (THU). Pandora Pagellus erythrinus (PAG), large red mullet (MUL3) and large pelagic fishes (LPL) also suffered high exploitation rates (Fig 5). Globally, the demersal domain resulted the most directly impacted by fishing activities.
Fig 5. Direct effects of fishing on the 3 domains: Exploitation rate (E) by FGs (left) and cumulative exploitation rate (Cum E) by fleet (right).
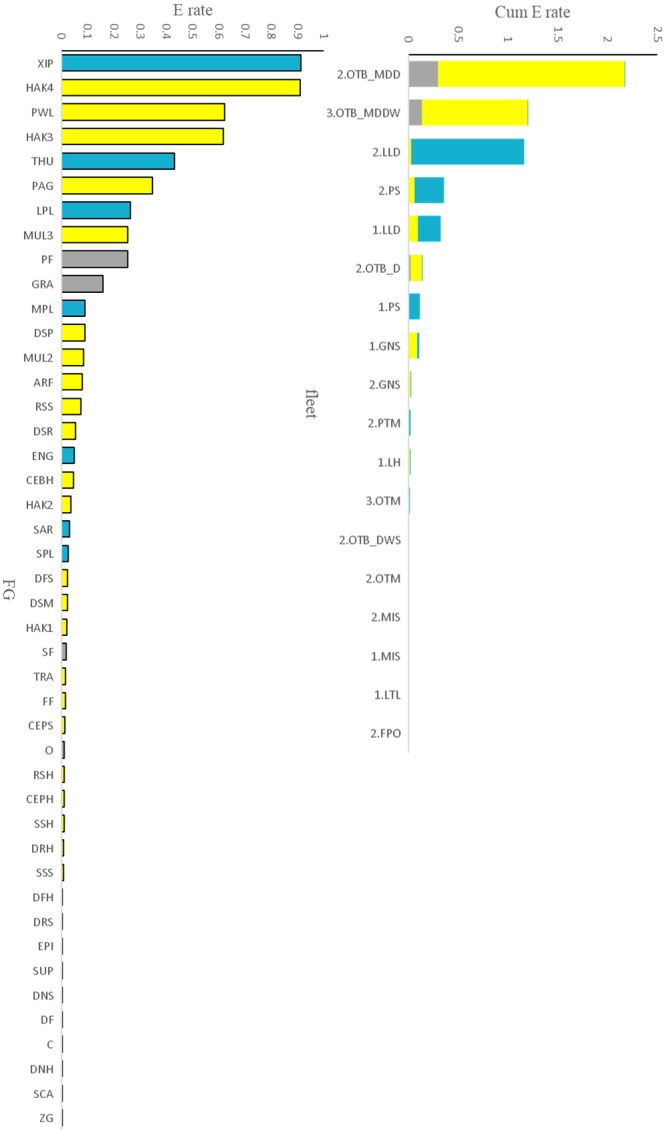
FGs belonging to pelagic (cyan), demersal (yellow) and benthic (gray) domain. Details of fleets and FGs in Tables 1–2.
Overall trophic relationships and FGs relevant for BPC
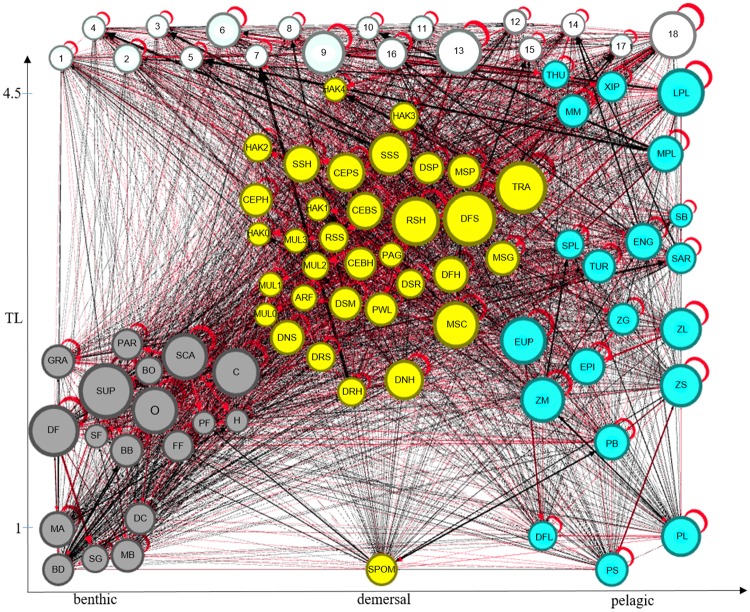
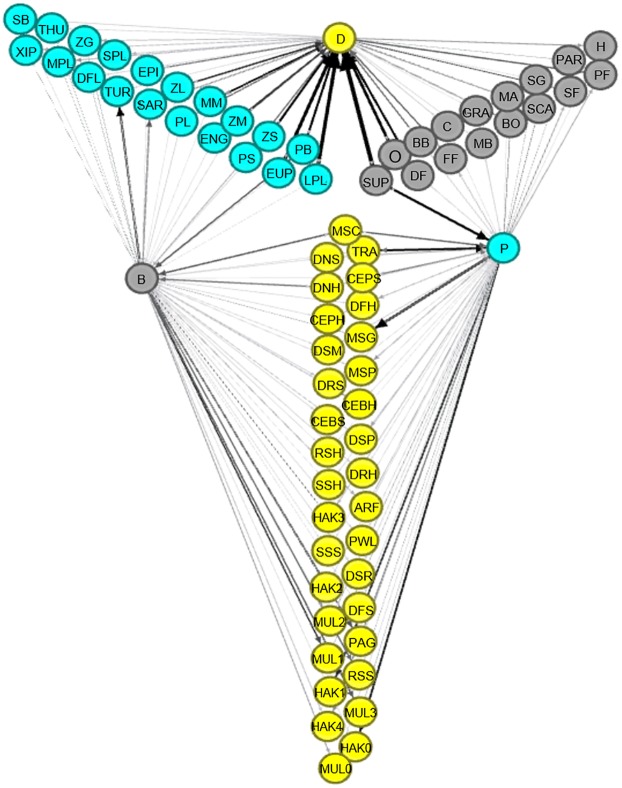
The results of the MTI analysis are synthetized in Fig 6. High values of ɛ (represented by node size) resulted for groups in all three domains. In the benthic domain, groups with high ɛi were suprabenthos (SUP), macrobenthos carnivore (C), omnivore (O), scavenger (SCA) and detritus feeder (DF); in the demersal domain, high ɛi values were shown by demersal fish from slope (DFS), TRA, MSC, rays from shelf (RSH), sharks from slope (SSS) and decapods natant from the slope (DNS). Finally in the pelagic domain, large pelagic fish (LPL), euphausiids (EUP), European anchovy (ENG), zooplankton medium (ZM) and small (ZS) were the groups with highest ɛi (Fig 6). These groups exerted high overall cumulative impacts through a large set of small impacts (low values of MTI; see also S2 Fig), while medium pelagic fish (MPL), demersal piscivorous fish (DSP) and reptant decapods from shelf (DRH) were the groups with the highest single value of MTI. The main overall effect on BPC was exerted by a group of key actors as shown in Fig 7. In fact, considering both direct and indirect overall effects, SUP, LPL, EUP produced high impacts on groups pertaining to other domains, while mesopelagic fish jellyfish feeders (MSG), SAR and sea turtles (TUR) were primarily affected by couplers of benthic and pelagic domains (Fig 7). Generally, macro-benthic organisms had a positive impact on demersal (e.g. SUP on HAK) and pelagic groups (e.g. SUP on SAR). Overall, the main impacting FGs on the BPC spanned from benthic (e.g., SUP) to pelagic (e.g., LPL) and exerted their impact mainly through demersal FGs (Fig 7).
Fig 6. Direct and indirect effects in the SoS food web.
Black arrows are positive effects, red arrows are negative effects. Arrow thickness is proportional to MTI (min = 0.03, max = 0.95). The 72 FGs are distinguished into main domain (benthic, demersal, pelagic) and by TL. Their overall cumulative effect in the web is proportional to the dimension of the circle.
Fig 7. BPC explored by overall impact among 69 living functional groups.
FGs are displaced on radial axes representing the domain which they belong to: pelagic (P, cyan), demersal (D, yellow), benthic (B, gray). Arrow thickness is proportional to the overall impact they have on the remaining domains as well as the impact FGs received by the domains (size nodes) they do not belong to. Higher overall impact (black arrow) are from the most impacting FGs to side nodes and from side nodes to the most impacted FGs detailed in Table 2.
BPC and mixed fisheries relationship
The MTI analysis showed fishing effects cascading on the food web (Fig 8A) as well as overall interactions among fleets (Fig 8B).
Fig 8. Results of the MTI analysis for fleets of the SoS ecosystem model.
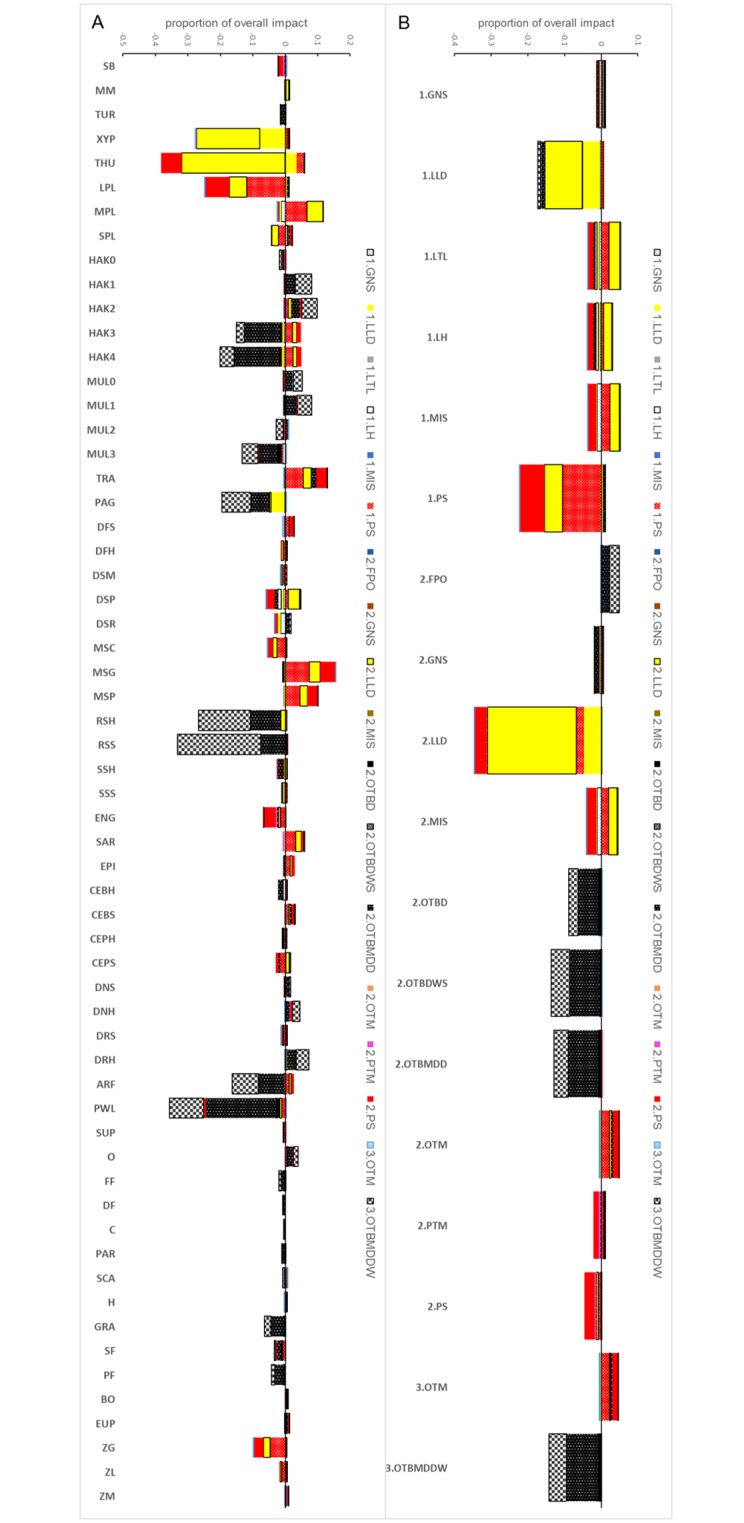
(A) overall impact across biological functional groups. (B) conflicts (negative values of MTI) and benefits (positive values of MTI) among fleet segments.
Bottom trawlers of LOA class 2 and 3 (2.OTB_MDD, 3.OTB_MDDW) had negative direct impact on main target demersal species such as large European hake (HAK3 and 4), red mullet (MUL3), rays and sharks (RSH and RSS), but had positive effects on hake and red mullet juvenile (HAK1 and 2; MUL0 and 1). Moreover, these fisheries had negative indirect impact on benthic organisms such as benthic decapods (mainly DNH, DRH) mediated by the depletion of their predators (Fig 8A). Similarly, longliners (1.LLD, 2.LLD) and purse-seiners (1.PS, 2.PS) had negative effects on their main pelagic targets, i.e., the large pelagic (XIP, THU, LPL) but also and indirect positive effect on several demersal species such as TRA, MSG, MSP and notably, HAK3 and HAK4 (Fig 8B). Interestingly purse-seiners have negative impact on jellyfish (ZG) which is not among their target species. HAK and TRA were bycatch species of pelagic pair trawlers (PTM) and midwater-mixed trawlers (OTM) and were indirectly favored by purse-seiners (LOA1 and 2) through the BPC (Fig 8A).
The MTI disentangled by fleet (Fig 8B) shows that, besides the strong intra-gear competition (negative values), bottom trawlers had a small, indirect and positive effect on pair and midwater-mixed trawlers. A large negative impact of 2.OTB_MDD on other bottom trawlers was evident as well as a positive effect on traps. A competition (negative impact) between LOA1 and LOA2 of both purse-seiners (GNS) and long-liners (LLD) appeared but purse-seiners had positive effects on 2-3.OTM (Fig 8B).
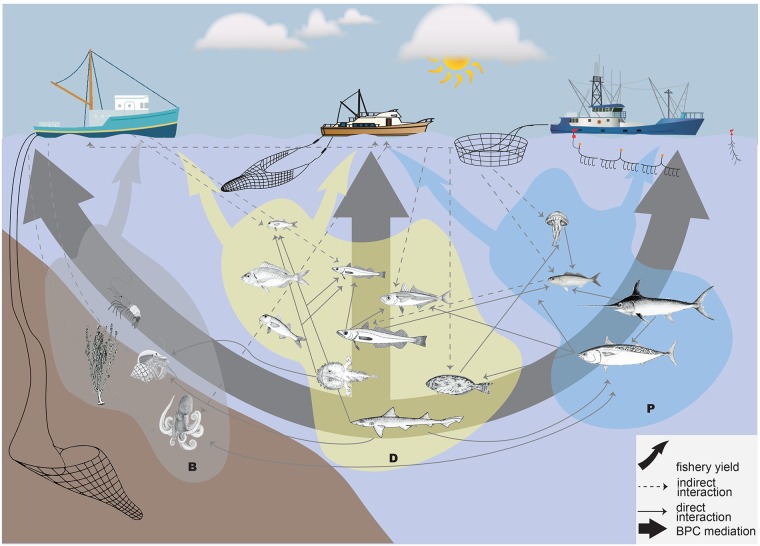
Fig 9 synthesizes the complex set of indirect interactions that connect bottom trawling to pelagic pair and midwater-mixed trawlers, showing that the positive effect of the bottom trawling on the latter is obtained promoting weak and diffused interactions that have a positive effect on TRA (e.g. sharks-large and medium pelagic fish-cephalopods-TRA). Similarly, the negative effect of purse seiners and long-liners on medium (MPL) and large pelagic fish (LPL), promotes an increase in jellyfish feeders such as EPI (mainly Boops boops) and MSG (mainly Schedophilus medusophagus) and a decrease in jellyfish (ZG). At the same time, biomass of predators such as benthic cephalopods from slope (CEBS), mesopelagic piscivorous fish (MSP) and DFS is released. However, forage fish and horse mackerel populations can increase so that predators such as European hake could benefit from this food resource (Fig 9).
Fig 9. BPC mediates mixed fisheries: Main fleet and biological overall impacts (direct and indirect) through the three domains.
Gray cloud = benthic domain, yellow cloud = demersal domain, blue cloud = pelagic domain. From left to right the fleets represent: bottom trawl, pair and mid-water trawl, purse seine and longline.
Discussion
Model validation
The development of the SoS ecosystem model required reconciling data and parameters obtained from a variety of independent sources and for a very wide set of species. The efforts for producing an accurate ecosystem description started with the revision and comparison of different data sources but unavoidably encompassed adjustments with respect to data which were the smallest possible. The comparison between Ecopath inputs and parameters with those of stock assessments highlighted a good accuracy of the ecosystem model developed. The comparison also evidenced that, in order to have similar total mortalities in the two approaches, it is often resulting in higher natural mortality in Ecopath possibly for accommodating predation mortalities. Conversely fishing mortalities for target species resulted slightly smaller. The review of wide set of information regarding biological, domain and fisheries aspects for the development of SoS model highlighted local gaps (such as the information on the benthic infauna) and, general issues encountered when working in a context of multiple targets and mixed fisheries, such as the difficulties in defining a model area that overlap completely resources, exploitations and data available.
Ecosystem structure and BPC
The food web of the Strait of Sicily described by our model resulted quite complex as suggested by the System Omnivory Index (SOI = 0.30), which is way above the average of 105 food web models [72] and other Mediterranean Ecopath models [68–70,75,76]. The SOI is considered a robust indicator in network analysis and its fluctuations should not be significantly influenced by the number of groups considered in the model [77]. Furthermore, Polis & Strong (1996) evidenced two main effects of omnivory: firstly, it diffuses the effects of productivity and consumption on the range of the trophic spectrum, secondly, consumers can increase by feeding on occasional prey, thus triggering a depression of their usual food sources. The first process could end up with a reinforced coupling across domains (i.e. benthic-pelagic) and the second could promote cascade effects.
The results have evidenced that most of the food web energetic fluxes were exchanged between bacteria and the various type of detritus (BD, SPOM, DC) and then, transferred from the detritus to the upper trophic levels. Bacteria and invertebrates of benthic and pelagic domains are characterized by high rate of production and consumption so that they handled the bulk of fluxes directly and indirectly linked to detritus. Thus, through a variety of feeding strategies, they were able to efficiently use detritus allowing an important coupling between planktonic and benthic invertebrates. Accordingly, other authors have suggested a prominent role of particulate organic matter, especially for marine snow (here SPOM) [9] across the whole vertical axis of the Mediterranean food web.
Moreveor, we have shown that high and medium trophic level items such as large pelagic fish (LPL), mesopelagic fish (MSC, MSP, MSG), Trachurus spp. (TRA), rays, sharks, cephalopods and demersal fish (DFH) had high ranks of overall effects. Medium pelagic (MPL), demersal piscivores (DSP) and reptant decapods from shelf (DRH) have high MTI values and fish that undergo through size/age related diet shifts (here red mullet and European hake) interact with a large number of prey and predators from all domains deeply contributing to BPC in space [78]. Most of these FGs indeed, are able to move on a large spatial scale and their life history is spent between shelf and slope domains where they play a key role in linking subsystems together (i.e. shelf-slope) at a wide scale [78,79] making ultimately the ecosystem more stable [78,80]. Moreover, most trophic interactions among components were low in terms of energetic flux but relevant for the number of links suggesting the existence of a large set of weak interactions. A primary importance has been given to this type of interactions in bio-diverse systems [79,81] because weak interactions serve to limit energy flow in a potentially strong consumer—resource interaction and, therefore, to inhibit over-consumption that destabilizes the dynamics of food webs [79,82].
Role of ecosystem components on the BPC
The SoS has been an important fishing ground since ancient times and, at the same time it represents an ecosystem that largely contributes to Mediterranean biodiversity in terms of habitats and species [38]. Critically enough, however, several target species such as deep-water rose shrimp (PWL), hake (HAK) and swordfish (XIP) resulted overexploited and many non-commercial species suffer conspicuous fishing mortality mainly by large bottom trawlers, long-liners and purse-seiners. Furthermore, a high discard ratio (0.45), in particular benthic invertebrates and horse mackerel, resulted associated to bottom trawlers [63,83].
Our results show that fishery driven interactions are involved in BPC through their pervasive action on the whole food web and through indirect effects among fleets. Acting on resources linked by weak interactions, the fleets possibly promote cascade effects such as those shown here for the Strait of Sicily but possibly observable in other Mediterranean ecosystems.
The model proposed in this study does not include processes and effects linked to the habitat modification caused by bottom trawling as well as the effects of physical factors (i.e. temperature, marine currents, etc.) and the mass balance can facilitate the identification of main contributors, but their consistency may be strengthened in a next step by implementing the temporal dynamic approach i.e., the Ecosim module [84,85]. However, the MTI analysis is a proxy of a dynamic simulation [74] and some steps of the important interactions reported here (i.e. trawlers-mega_macrobenthos, epipelagic fish- jellyfish) have also been demonstrated by other authors in the Mediterranean [86–89]. Moreover, several benthic FGs such as suspension and filter feeders appeared negatively affected by trawling. These FGs are habitat formers and have been suggested to have a key role in BPC and in the carbon cycle [90], and are also vulnerable to fishing. In fact, filter feeders such as the deep sea corals Isidella elongata and Funiculina quadrangularis are critically endangered by trawlers in the SoS [91].
Benthic-pelagic coupling therefore appears to be shaped by multiple interactions among group of species (sp.-sp.), among fleets (fleet-fleet) as well as between fleet and group of species (fleet-sp.). Although the analysis of direct interactions suggests that main elements of BPC are mediated by wasp-waist groups (mainly mesopelagic fish crustacean feeders, MSC and epipelagic fish, EPI), which show great contribution to energy transfer as consumers and sources in the food web, the quantification of direct and indirect impacts highlighted the important role of other components in the BPC.
Interestingly, it has been recently suggested that the increasing depletion of top predators over the past decades, has led to a shift of fisheries towards forage fish and benthic invertebrates [92] that could have an important role in BPC [26]. Forage fish have been also recognized as wasp-waist species [93] exerting a double important role: top-down on their prey and bottom-up on their predators. The MTI and fluxes shown here suggest that many groups over wasp-waist species could play a role in the BPC regardless of which domain they belong to. Moreover, most of the target species are linked by weak interactions and the high erosion of biomass could make them highly sensitive to fishing and may lead to the rise of unexpected consumers [94] and trophic cascades into a scenario of simplified and degraded ecosystems.
Moreover, the result of this study shows that BPC mediates the Mediterranean mixed fishery through an intricate combination of biological and fisheries interactions and therefore the idea of unrelated demersal and pelagic fisheries is to be set aside.
Overall, this study provides a novel and comprehensive quantitative integration of a large amount of information on biomass density, population dynamics and trophic interactions in the Strait of Sicily, contributing to a deeper understanding of the functioning of the whole food web, and offers a novel assessment of the overall effects of fishing in an ecological Mediterranean framework.
Supporting information
(DOCX)
Biomass (B); diet information used to build the diet matrix; production per unit of biomass (P/B); consumption per unit of biomass (Q/B). F = fishing mortality, M = natural mortality, Z = total mortality.
(DOCX)
For each functional group (FG) are detailed inputs: biomass (B; t km−2), production/biomass ratio (P/B; yr−1), consumption/biomass ratio (Q/B; yr−1); Landing and Discards are expressed in t km−2 year−1. Outputs: trophic level (TL), ecotrophic efficiency (EE; for most of the FG except for BO, EUP, MB), production/consumption (P/Q), respiration/assimilation (R/A), omnivory index (OI). Dom = Domain: p = pelagic, d = demersal, b = benthic.
(DOCX)
Raw data across functional groups of predators in columns and preys in rows.
(DOCX)
B total biomass (exluding detritus), Q total consumption, Ex sum of all exports, R sum of all respiratory flow, FD sum of all flows into detritus, P sum of all productions, TST total system throughput, PP net primary production, TE mean transfer efficiency, C capacity, O overhead, A ascendency, FCI model stress, Y catches, TLm mean trophic level of catches, Y/PP gross efficiency.
(DOCX)
Proportion of preys (rows) in the diet of the predators (columns) in ranks. Fraction of import in the diet (fraction of energy assumed to be taken out of the system) are also indicated.
(TIF)
Rows are impacting FGs and columns impacted FGs.
(TIF)
Acknowledgments
This work has been funded by the Flagship Project RITMARE—The Italian Research for the Sea—coordinated by the Italian National Research Council and funded by the Italian Ministry of Education, University and Research within the National Research Program 2011–2013. Many thanks to Valentina Mosetti for precious help in drawing the pictogram.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work has been funded by the Flagship Project RITMARE - The Italian Research for the Sea - coordinated by the Italian National Research Council and funded by the Italian Ministry of Education, University and Research within the National Research Program 2011-2013 to DA.
References
- 1.Lee J, Tett P, Jones K, Jones S, Luyten P, Smith C, et al. The PROWQM physical—biological model with benthic—pelagic coupling applied to the northern North Sea. J Sea Res. 2002;48: 287–331. 10.1016/S1385-1101(02)00182-X [DOI] [Google Scholar]
- 2.Dunton KH, Goodall JL, Shonberg SV, Grebmeier JM, Maichment DR. Multi-decadal synthesis of bentho-pelagic coupling in the western arctic: role of cross-shelf advictive processes. Deep Res II. 2005;52: 3462–3477. 10.1016/j.dsr2.2005.09.007 [DOI] [Google Scholar]
- 3.Marcus NH, Boero F. Minireview: The importance of benthic-pelagic coupling and the forgotten role of life cycles in coastal aquatic systems. Limnol Oceanogr. 1998;43: 763–768. 10.4319/lo.1998.43.5.0763 [DOI] [Google Scholar]
- 4.Griffiths JR, Kadin M, Nascimento FJA, Tamelander T, Törnroos A, Bonaglia S, et al. The importance of benthic-pelagic coupling for marine ecosystem functioning in a changing world. Glob Chang Biol. 2017;23: 2179–2196. 10.1111/gcb.13642 [DOI] [PubMed] [Google Scholar]
- 5.Graf G, Rosenberg R. Bioresuspension and biodeposition: A review. J Mar Syst. 1997;11: 269–278. 10.1016/S0924-7963(96)00126-1 [DOI] [Google Scholar]
- 6.Raffaelli D, Bell E, Weithoff G, Matsumoto A, Cruz-Motta JJ, Kershaw P, et al. The ups and downs of benthic ecology: Considerations of scale, heterogeneity and surveillance for benthic-pelagic coupling. Journal of Experimental Marine Biology and Ecology. 2003. pp. 191–203. 10.1016/S0022-0981(02)00527-0 [DOI] [Google Scholar]
- 7.Polunin NVC, Morales-Nin B, Pawsey WE, Cartes JE, Pinnegar JK, Moranta J. Feeding relationships in Mediterranean bathyal assemblages elucidated by stable nitrogen and carbon isotope data. Mar Ecol Prog Ser. 2001;220: 13–23. 10.3354/meps220013 [DOI] [Google Scholar]
- 8.Pinnegar JK, Polunin NVC, Videler JJ, de Wiljes JJ. Daily carbon, nitrogen and phosphorus budgets for the Mediterranean planktivorous damselfish Chromis chromis. J Exp Mar Bio Ecol. 2007;352: 378–391. 10.1016/j.jembe.2007.08.016 [DOI] [Google Scholar]
- 9.Tecchio S, Coll M, Sardà F. Structure, functioning, and cumulative stressors of Mediterranean deep-sea ecosystems. Prog Oceanogr. 2015;135: 156–167. 10.1016/j.pocean.2015.05.018 [DOI] [Google Scholar]
- 10.Bollens SM, Frost BW, Thoreson DS, Watts SJ. Diel vertical migration in zooplankton: field evidence in support of the predator avoidance hypothesis. Hydrobiologia. 1992;234: 33–39. 10.1007/BF00010777 [DOI] [Google Scholar]
- 11.Folt C, Burns C. Biological drivers of zooplankton patchiness. Trends in Ecology and Evolution. 1999. pp. 300–305. 10.1016/S0169-5347(99)01616-X [DOI] [PubMed] [Google Scholar]
- 12.McCormick MI. Ontogeny of diet shifts by a microcarnivorous fish, Cheilodactylus spectabilis: Relationship between feeding mechanics, microhabitat selection and growth. Mar Biol. 1998;132: 9–20. 10.1007/s002270050367 [DOI] [Google Scholar]
- 13.Lauzon-Guay JS, Scheibling RE. Behaviour of sea urchin Strongylocentrotus droebachiensis grazing fronts: Food-mediated aggregation and density-dependent facilitation. Mar Ecol Prog Ser. 2007;329: 191–204. 10.3354/meps329191 [DOI] [Google Scholar]
- 14.Sánchez-Hernández J, Eloranta AP, Finstad AG, Amundsen PA. Community structure affects trophic ontogeny in a predatory fish. Ecol Evol. 2017;7: 358–367. 10.1002/ece3.2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abid N, Laglaoui A, Arakrak A, Bakkali M. The role of fish in the diet of swordfish (Xiphias gladius) in the Strait of Gibraltar. J Mar Biol Assoc United Kingdom. 2017; 1–13. 10.1017/S002531541700011X [DOI] [Google Scholar]
- 16.Sinopoli M, Fanelli E, D’Anna G, Badalamenti F, Pipitone C. Assessing the effects of a trawling ban on diet and trophic level of hake, Merluccius merluccius, in the southern Tyrrhenian Sea. Sci Mar. 2012;76: 677–690. 10.3989/scimar.03564.29A [DOI] [Google Scholar]
- 17.Baustian MM, Hansen GJ a., de Kluijver A, Robinson K, Henry EN, Knoll LB, et al. Linking the bottom to the top in aquatic ecosystems: mechanisms and stressors of benthic-pelagic coupling. Eco-DAS X Symp Proc. 2014; 25–47. [Google Scholar]
- 18.Puig P, Canals M, Company JB, Martín J, Amblas D, Lastras G, et al. Ploughing the deep sea floor. Nature. 2012;489: 286–289. 10.1038/nature11410 [DOI] [PubMed] [Google Scholar]
- 19.Tecchio S, Coll M, Christensen V, Company JB, Ramírez-Llodra E, Sardà F. Food web structure and vulnerability of a deep-sea ecosystem in the NW Mediterranean Sea. Deep Res Part I Oceanogr Res Pap. 2013;75: 1–15. 10.1016/j.dsr.2013.01.003 [DOI] [Google Scholar]
- 20.Shannon L, Coll M, Bundy A, Gascuel D, Heymans JJ, Kleisner K, et al. Trophic level-based indicators to track fishing impacts across marine ecosystems. Mar Ecol Prog Ser. 2014;512: 115–140. 10.3354/meps10821 [DOI] [Google Scholar]
- 21.Sciberras M, Hiddink JG, Jennings S, Szostek CL, Hughes KM, Kneafsey B, et al. Response of benthic fauna to experimental bottom fishing: A global meta-analysis. Fish Fish. 2018;19: 698–715. 10.1111/faf.12283 [DOI] [Google Scholar]
- 22.Howarth L, Waggitt J, Bolam S, Eggleton J, Somerfield P, Hiddink J. Effects of bottom trawling and primary production on the composition of biological traits in benthic assemblages. Mar Ecol Prog Ser. 2018;602: 31–48. 10.3354/meps12690 [DOI] [Google Scholar]
- 23.Hiddink JG, Jennings S, Sciberras M, Szostek CL, Hughes KM, Ellis N, et al. Global analysis of depletion and recovery of seabed biota after bottom trawling disturbance. Proc Natl Acad Sci. 2017;114: 8301–8306. 10.1073/pnas.1618858114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celic I, Libralato S, Scarcella G, Raicevich S, Marceta B, Solidoro C. Ecological and economic effetcs of the landing obligation evaluated using a quantitative ecosystem approach: a mediterranean case study. ICES J Mar Sci. 2018. [Google Scholar]
- 25.Eigaard OR, Bastardie F, Hintzen NT, Buhl-Mortensen L, Buhl-Mortensen P, Catarino R, et al. The footprint of bottom trawling in European waters: Distribution, intensity, and seabed integrity. ICES J Mar Sci. 2017;74: 847–865. 10.1093/icesjms/fsw194 [DOI] [Google Scholar]
- 26.Eddy TD, Lotze HK, Fulton EA, Coll M, Ainsworth CH, de Araújo JN, et al. Ecosystem effects of invertebrate fisheries. Fish Fish. 2017;18: 40–53. 10.1111/faf.12165 [DOI] [Google Scholar]
- 27.Kaiser MJ, Clarke KR, Hinz H, Austen MCV, Somerfield PJ, Karakassis I. Global analysis of response and recovery of benthic biota to fishing. Mar Ecol Prog Ser. 2006;311: 1–14. [Google Scholar]
- 28.Pusceddu A, Bianchelli S, Martin J, Puig P, Palanques A, Masque P, et al. Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. Proc Natl Acad Sci. 2014;111: 8861–8866. 10.1073/pnas.1405454111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin YJ, Rochet MJ, Jennings S, Field JG, Gislason H. Using size-based indicators to evaluate the ecosystem effects of fishing. ICES Journal of Marine Science. 2005. pp. 384–396. 10.1016/j.icesjms.2005.01.004 [DOI] [Google Scholar]
- 30.Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, et al. The Rise of the Mesopredator. Bioscience. 2009;59: 779–791. 10.1525/bio.2009.59.9.9 [DOI] [Google Scholar]
- 31.Pikitch EK, Santora C, Babcock EA, Bakun A, Bonfil R, Conover DO, et al. Ecology. Ecosystem-based fishery management. Science. American Association for the Advancement of Science; 2004;305: 346–7. 10.1126/science.1098222 [DOI] [PubMed] [Google Scholar]
- 32.Petihakis G, Smith CJ, Triantafyllou G, Sourlantzis G, Papadopoulou KN, Pollani A, et al. Scenario testing of fisheries management strategies using a high resolution ERSEM-POM ecosystem model. ICES J Mar Sci. 2007;64: 1627–1640. 10.1093/icesjms/fsm161 [DOI] [Google Scholar]
- 33.Christensen V, Walters CJ. Ecopath with Ecosim: Methods, capabilities and limitations. Ecological Modelling. 2004. pp. 109–139. 10.1016/j.ecolmodel.2003.09.003 [DOI] [Google Scholar]
- 34.Fulton EA, Link JS, Kaplan IC, Savina-Rolland M, Johnson P, Ainsworth C, et al. Lessons in modelling and management of marine ecosystems: The Atlantis experience. Fish Fish. 2011;12: 171–188. 10.1111/j.1467-2979.2011.00412.x [DOI] [Google Scholar]
- 35.Travers-Trolet M, Shin YJ, Shannon LJ, Moloney CL, Field JG. Combined fishing and climate forcing in the southern Benguela upwelling ecosystem: An end-to-end modelling approach reveals dampened effects. Álvarez I, editor. PLoS One. 2014;9: e94286 10.1371/journal.pone.0094286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coll M, Libralato S. Contributions of food web modelling to the ecosystem approach to marine resource management in the Mediterranean Sea. Fish Fish. 2012;13: 60–88. 10.1111/j.1467-2979.2011.00420.x [DOI] [Google Scholar]
- 37.FAO. The State of Mediterranean and Black Sea Fisheries. General Fisheries Commission for the Mediterranean, Rome. 2016.
- 38.Di Lorenzo M, Sinerchia M, Colloca F. The North sector of the Strait of Sicily: a priority area for conservation in the Mediterranean Sea. Hydrobiologia. Springer International Publishing; 2017; 1–19. 10.1007/s10750-017-3389-7 [DOI] [Google Scholar]
- 39.Fiorentino F, Ben Hadj Hamida O, Ben Meriem S, Gaamour A, Gristina M, Jarboui O, et al. Synthesis of information on some demersal crustaceans relevant for fisheries target species in the south-central Mediterranean Sea. MedSudMed Tech. Doc 32: 120 pp. 2013. [Google Scholar]
- 40.Knittweiss L, Arneri E, Ben Meriem S, Dimech M, Fiorentino F, Gancitano V, et al. Stock status and potential yield of deep water rose shrimp (Parapenaeus longirostris, Lucas 1846) in the south-central Mediterranean Sea. MedSudMed Tech. Doc, 28: 15 pp. 2013. [Google Scholar]
- 41.Civile D, Lodolo E, Zecchin M, Ben-Avraham Z, Baradello L, Accettella D, et al. The lost Adventure Archipelago (Sicilian Channel, Mediterranean Sea): Morpho-bathymetry and Late Quaternary palaeogeographic evolution. Glob Planet Change. 2015;125: 36–47. 10.1016/j.gloplacha.2014.12.003 [DOI] [Google Scholar]
- 42.Astraldi M, Gasparini GP, Gervasio L, Salusti E. Dense Water Dynamics along the Strait of Sicily (Mediterranean Sea). J Phys Oceanogr. 2001;31: 3457–3475. [DOI] [Google Scholar]
- 43.Béranger K, Mortier L, Gasparini GP, Gervasio L, Astraldi M, Crépon M. The dynamics of the Sicily Strait: A comprehensive study from observations and models. Deep-Sea Research Part II: Topical Studies in Oceanography. 2004. pp. 411–440. 10.1016/j.dsr2.2003.08.004 [DOI] [Google Scholar]
- 44.García Lafuente J, García A, Mazzola L, Quintanilla J, Delgado J, Cuttita A, et al. Hydrographic phenomena influencing early life stages of the Sicilian Channel Anchovy. Fish Oceanogr. 2002;11: 31–44. 10.1046/j.1365-2419.2002.00186.x [DOI] [Google Scholar]
- 45.Patti B, Guisande C, Bonanno A, Basilone G, Cuttitta A, Mazzola S. Role of physical forcings and nutrient availability on the control of satellite-based chlorophyll a concentration in the coastal upwelling area of the Sicilian Channel. Sci Mar. 2010;74: 577–588. 10.3989/scimar.2010.74n3577 [DOI] [Google Scholar]
- 46.Fortibuoni T, Bahri T, Camilleri M, Garofalo G, Gristina M, Fiorentino F. Nursery and Spawning Areas of Deep-water Rose Shrimp, Parapenaeus longirostris (Decapoda: Penaeidae), in the Strait of Sicily (Central Mediterranean Sea). J Crustac Biol. 2010;30: 167–174. 10.1651/09-3167.1 [DOI] [Google Scholar]
- 47.Garofalo G, Fortibuoni T, Gristina M, Sinopoli M, Fiorentino F. Persistence and co-occurrence of demersal nurseries in the Strait of Sicily (central Mediterranean): Implications for fishery management. J Sea Res. 2011;66: 29–38. 10.1016/j.seares.2011.04.008 [DOI] [Google Scholar]
- 48.Basilone G, Bonanno A, Patti B, Mazzola S, Barra M, Cuttitta A, et al. Spawning site selection by European anchovy (Engraulis encrasicolus) in relation to oceanographic conditions in the Strait of Sicily. Fish Oceanogr. 2013;22: 309–323. 10.1111/fog.12024 [DOI] [Google Scholar]
- 49.Torri M, Corrado R, Falcini F, Cuttitta A, Palatella L, Lacorata G, et al. Planktonic stages of small pelagic fishes (Sardinella aurita and Engraulis encrasicolus) in the central Mediterranean Sea: The key role of physical forcings and implications for fisheries management. Prog Oceanogr. 2018;162: 25–39. 10.1016/j.pocean.2018.02.009 [DOI] [Google Scholar]
- 50.Garofalo G, Fiorentino F, Gristina M, Cusumano S, Sinacori G. Stability of spatial pattern of fish species diversity in the Strait of Sicily (central Mediterranean). Hydrobiologia. 2007. pp. 117–124. 10.1007/s10750-006-0460-1 [DOI] [Google Scholar]
- 51.Bertrand JA, Gil de Sola L, Papaconstantinou C, Relini G, Souplet A, Souplet A. The general specifications of the MEDITS surveys. Sci March 2002;66: 9 10.3989/scimar.2002.66s29 [DOI] [Google Scholar]
- 52.Scarcella G, Grati F, Raicevich S, Russo T, Gramolini R, Scott RD, et al. Common sole in the northern and central Adriatic Sea: Spatial management scenarios to rebuild the stock. J Sea Res. 2014;89: 12–22. 10.1016/j.seares.2014.02.002 [DOI] [Google Scholar]
- 53.Lopez S. L’ecosistema del Mar Tirreno: aspetti strutturali, funzionali, effetti della pesca e delle interazioni trofiche. PhD thesis—University of Rome “La Sapienza.” 2013.
- 54.Jul-Larsen E, Kolding J, Overå R, Nielsen JR, van Zwieten PAM. FAO Fisheries Technical Paper 426/1-Management, Co-Management or No Management? 2003.
- 55.Fiorentino F, Patti B, Colloca F, Bonanno A, Basilone G, Gancitano V, et al. A comparison between acoustic and bottom trawl estimates to reconstruct the biomass trends of sardine and anchovy in the Strait of Sicily (Central Mediterranean). Fish Res. 2013;147: 290–295. 10.1016/j.fishres.2013.06.001 [DOI] [Google Scholar]
- 56.Mazzola S, Patti B, Bonanno A. AMECO-Scienze marine applicate alla gestione delle risorse rinnovabili del mare: il case study della popolazione di alici nello Stretto di Sicilia. MIUR final report. 2006.
- 57.Romano C, Fanelli E, D’Anna G, Pipitone C, Vizzini S, Mazzola A, et al. Spatial variability of soft-bottom macrobenthic communities in northern Sicily (Western Mediterranean): Contrasting trawled vs. untrawled areas. Mar Environ Res. 2016;122: 113–125. 10.1016/j.marenvres.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 58.Lazzari P, Solidoro C, Ibello V, Salon S, Teruzzi A, Béranger K, et al. Seasonal and inter-annual variability of plankton chlorophyll and primary production in the Mediterranean Sea: A modelling approach. Biogeosciences. 2012;9: 217–233. 10.5194/bg-9-217-2012 [DOI] [Google Scholar]
- 59.ICCAT, editor. Collective Volume of Scientific Papers—International Commission for the Conservation of Atlantic Tunas ICCAT editor. Madrid, Spain; 2010.
- 60.Block BA, Dewar H, Blackwell SB, Williams TD, Prince ED, Farwell CJ, et al. Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science (80-). 2001;293: 1310–1314. 10.1126/science.1061197 [DOI] [PubMed] [Google Scholar]
- 61.Sarà G, Sarà R. Feeding habits and trophic levels of bluefin tuna Thunnus thynnus of different size classes in the Mediterranean Sea. J Appl Ichthyol. 2007;23: 122–127. 10.1111/j.1439-0426.2006.00829.x [DOI] [Google Scholar]
- 62.Garofalo G, Giusti GB, Cusumano S, Igrande G, Sinacori G, Gristina M, et al. Catch Per Unit Effort of Red Shrimp in Bathyal Fishing Grounds Fo the Eastern Mediterranean. Biol Mar Mediterr. 2007;14: 250–251. [Google Scholar]
- 63.Milisenda G, Vitale S, Massi D, Enea M, Ganci V, Giusto GB, et al. Discard composition associated with the deep water rose shrimp fisheries (Parapenaeus longirostris, Lucas 1846) in the south-central Mediterranean Sea. Mediterr Mar Sci. 2016; 10.12681/mms.1787 [DOI] [Google Scholar]
- 64.Levi D, Gristina M, Norrito G. Analysis of trawls’ discard operation in the central and eastern Mediterranean Sea. Final report. 1997.
- 65.Castriota L, Campagnuolo S, Andaloro F. Shrimp Trawl Fishery By-catch in the Straits of Sicily (Central Mediterranean Sea). Sci Counc Meet—NAFO SCR Doc 01/113. 2001; 9. [Google Scholar]
- 66.Mannini A, Sabatella F. Annuario sullo Stato delle Risorse e sulle Strutture Produttive dei Mari Italiani. Biol Mar Mediterr. 2015;22: 358. [Google Scholar]
- 67.Milisenda G, Vitale S, Massi D, Enea M, Gancitano V, Giusto GB, et al. Spatio-temporal composition of discard associated with the deep water rose shrimp fisheries (Parapenaeus longirostris, Lucas 1846) in the south-central Mediterranean Sea. Mediterr Mar Sci. 2017;18: 53–63. [Google Scholar]
- 68.Tsagarakis K, Coll M, Giannoulaki M, Somarakis S, Papaconstantinou C, Machias A. Food-web traits of the North Aegean Sea ecosystem (Eastern Mediterranean) and comparison with other Mediterranean ecosystems. Estuar Coast Shelf Sci. 2010;88: 233–248. 10.1016/j.ecss.2010.04.007 [DOI] [Google Scholar]
- 69.Moutopoulos DK, Libralato S, Solidoro C, Stergiou KI. Toward an ecosystem approach to fisheries in the Mediterranean Sea: Multi-gear/multi-species implications from an ecosystem model of the Greek Ionian Sea. J Mar Syst. 2013;113–114: 13–28. 10.1016/j.jmarsys.2012.12.002 [DOI] [Google Scholar]
- 70.Piroddi C, Coll M, Steenbeek J, Moy DM, Christensen V. Modelling the Mediterranean marine ecosystem as a whole: Addressing the challenge of complexity. Mar Ecol Prog Ser. 2015;533: 47–65. 10.3354/meps11387 [DOI] [Google Scholar]
- 71.Libralato S, Coll M, Tempesta M, Santojanni A, Spoto M, Palomera I, et al. Food-web traits of protected and exploited areas of the Adriatic Sea. Biol Conserv. 2010;143: 2182–2194. 10.1016/j.biocon.2010.06.002 [DOI] [Google Scholar]
- 72.Libralato S. Trophic amplitude as an indicator of aquatic food web complexity. Vie Milieu—Life Environ. 2016;66: 315–323. [Google Scholar]
- 73.Ulanowicz RE, Puccia C. Mixed trophic impacts in ecosystems. Coenoses. 1990;5: 7–16. [Google Scholar]
- 74.Libralato S, Christensen V, Pauly D. A method for identifying keystone species in food web models. Ecol Modell. 2006;195: 153–171. 10.1016/j.ecolmodel.2005.11.029 [DOI] [Google Scholar]
- 75.Coll M, Santojanni A, Palomera I, Tudela S, Arneri E. An ecological model of the Northern and Central Adriatic Sea: Analysis of ecosystem structure and fishing impacts. J Mar Syst. 2007;67: 119–154. 10.1016/j.jmarsys.2006.10.002 [DOI] [Google Scholar]
- 76.Coll M, Santojanni A, Palomera I, Arneri E. Food-web changes in the Adriatic Sea over the last three decades. Mar Ecol Prog Ser. 2009;381: 17–37. 10.3354/meps07944 [DOI] [Google Scholar]
- 77.Libralato S. System Omnivory Index. Encyclopedia of Ecology. 2008. pp. 3473–3477. [Google Scholar]
- 78.McCann KS, Rasmussen JB, Umbanhowar J. The dynamics of spatially coupled food webs. Ecol Lett. 2005;8: 513–523. 10.1111/j.1461-0248.2005.00742.x [DOI] [PubMed] [Google Scholar]
- 79.Polis GA, Strong DR. Food Web Complexity and Community Dynamics. Am Nat. 1996;147: 813–846. 10.1086/285880 [DOI] [Google Scholar]
- 80.Post DM, Conners ME, Goldberg DS, Jan N. Prey Preference by a Top Predator and the Stability of Linked Food Chains. Ecology. 2008;81: 8–14. 10.2307/177129 [DOI] [Google Scholar]
- 81.Berlow EL. Strong effects of weak interactions in ecological communities. Nature. Nature Publishing Group; 1999;398: 330–334. 10.1038/18672 [DOI] [Google Scholar]
- 82.McCann KS. The diversity—stability debate. Nature. 2000;405: 228–233. 10.1038/35012234 [DOI] [PubMed] [Google Scholar]
- 83.Consoli P, Esposito V, Falautano M, Battaglia P, Castriota L, Romeo T, et al. The impact of fisheries on vulnerable habitats: the case of trawling on circa-littoral grounds in the Strait of Sicily (central Mediterranean Sea). Mar Biol Res. 2017;13: 1084–1094. 10.1080/17451000.2017.1348010 [DOI] [Google Scholar]
- 84.Pauly D, Christensen V, Walters C. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J Mar Sci. 2000;57: 697–706. 10.1006/jmsc.2000.0726 [DOI] [Google Scholar]
- 85.Walters C, Christensen V. Adding realism to foraging arena predictions of trophic flow rates in Ecosim ecosystem models: Shared foraging arenas and bout feeding. Ecol Modell. 2007;209: 342–350. 10.1016/j.ecolmodel.2007.06.025 [DOI] [Google Scholar]
- 86.De Juan S, Thrush SF, Demestre M. Functional changes as indicators of trawling disturbance on a benthic community located in a fishing ground (NW Mediterranean Sea). Mar Ecol Prog Ser. 2007;334: 117–129. 10.3354/meps334117 [DOI] [Google Scholar]
- 87.Mangano M, Kaiser MJ, Porporato EMD, Spanò N. Evidence of trawl disturbance on mega-epibenthic communities in the southern tyrrhenian sea. Mar Ecol Prog Ser. 2013;475: 101–117. 10.3354/meps10115 [DOI] [Google Scholar]
- 88.Milisenda G, Rosa S, Fuentes VL, Boero F, Guglielmo L, Purcell JE, et al. Jellyfish as prey: Frequency of predation and selective foraging of boops boops (vertebrata, actinopterygii) on the mauve stinger pelagia noctiluca (cnidaria, scyphozoa). Hays GC, editor. PLoS One. 2014;9: e94600 10.1371/journal.pone.0094600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tilves U, Fuentes V, Milisenda G, Parrish C, Vizzini S, Sabatés A. Trophic interactions of the jellyfish Pelagia noctiluca in the NW Mediterranean: evidence from stable isotope signatures and fatty acid composition. Mar Ecol Prog Ser. 2018; 1–16. 10.3354/meps12332 [DOI] [Google Scholar]
- 90.Rossi S. The destruction of the “animal forests” in the oceans: Towards an over-simplification of the benthic ecosystems. Ocean Coast Manag. 2013;84: 77–85. 10.1016/j.ocecoaman.2013.07.004 [DOI] [Google Scholar]
- 91.Lauria V, Garofalo G, Fiorentino F, Massi D, Milisenda G, Piraino S, et al. Species distribution models of two critically endangered deep-sea octocorals reveal fishing impacts on vulnerable marine ecosystems in central Mediterranean Sea. Sci Rep. 2017;7: 8049 10.1038/s41598-017-08386-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith ADM, Brown CJ, Bulman CM, Fulton EA, Johnson P, Kaplan IC, et al. Impacts of fishing low-trophic level species on marine ecosystems. Science (80-). 2011;333: 1147–1150. 10.1126/science.1209395 [DOI] [PubMed] [Google Scholar]
- 93.Madigan DJ, Carlisle AB, Dewar H, Snodgrass OE, Litvin SY, Micheli F, et al. Stable Isotope Analysis Challenges Wasp-Waist Food Web Assumptions in an Upwelling Pelagic Ecosystem. Sci Rep. 2012;2: 654 10.1038/srep00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Damalas D, Maravelias CD, Osio GC, Maynou F, Sbrana M, Sartor P. “Once upon a time in the Mediterranean” long term trends of Mediterranean fisheries resources based on fishers’ traditional ecological knowledge. Gomez-Gesteira M, editor. PLoS One. 2015;10: e0119330 10.1371/journal.pone.0119330 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Biomass (B); diet information used to build the diet matrix; production per unit of biomass (P/B); consumption per unit of biomass (Q/B). F = fishing mortality, M = natural mortality, Z = total mortality.
(DOCX)
For each functional group (FG) are detailed inputs: biomass (B; t km−2), production/biomass ratio (P/B; yr−1), consumption/biomass ratio (Q/B; yr−1); Landing and Discards are expressed in t km−2 year−1. Outputs: trophic level (TL), ecotrophic efficiency (EE; for most of the FG except for BO, EUP, MB), production/consumption (P/Q), respiration/assimilation (R/A), omnivory index (OI). Dom = Domain: p = pelagic, d = demersal, b = benthic.
(DOCX)
Raw data across functional groups of predators in columns and preys in rows.
(DOCX)
B total biomass (exluding detritus), Q total consumption, Ex sum of all exports, R sum of all respiratory flow, FD sum of all flows into detritus, P sum of all productions, TST total system throughput, PP net primary production, TE mean transfer efficiency, C capacity, O overhead, A ascendency, FCI model stress, Y catches, TLm mean trophic level of catches, Y/PP gross efficiency.
(DOCX)
Proportion of preys (rows) in the diet of the predators (columns) in ranks. Fraction of import in the diet (fraction of energy assumed to be taken out of the system) are also indicated.
(TIF)
Rows are impacting FGs and columns impacted FGs.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.