Figure 1:
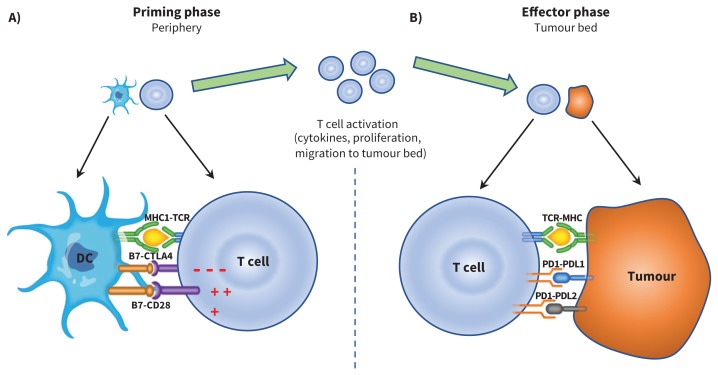
A) Antigen presenting cells (APC), such as dendritic cells (DC), scout the human body, sampling different antigens, including tumour-associated antigens, which are then processed and presented through the major histocompatibility (MHC) complex. In a priming phase that takes place in peripheral lymph nodes, APC educate and activate antigen-specific T cells. T cell activation is kept in check by cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), a negative regulator of T-cell function. This negative signal can be blocked by CTLA-4 inhibitors (i.e., ipilimumab), allowing ongoing T-cell activation and migration to the tumour bed. B) Activated T cells also express programmed cell death 1 (PD1), and its ligands, PDL1 and PDL2, are commonly expressed on APC. PD1 and PDL1/PDL2 interaction also inhibits T-cell responses. Tumours can express PDL1 and PDL2 receptors, and engagement of these receptors with the PD1 receptor on T cells turns off T cells and allows tumours to escape destruction. These immune checkpoints can be blocked by PD-1 inhibitors (i.e., nivolumab, pembrolizumab) or PDL-1 inhibitors (i.e., atezolizumab, avelumab and durvalumab). CTLA-4 inhibition results in more widespread and severe immune-related adverse events (irAE), as it regulates T-cell function early in the immunity cycle. Blocking PD-1, on the other hand, is more specific to the tumour microenvironment and, in general, results in less widespread and severe irAE. Note: CD28 = cluster of differentiation 28, TCR = T-cell receptor.