Abstract
Background
The adductor canal block (ACB) is an effective intervention for postoperative analgesia following total knee arthroplasty (TKA). However, the ideal ACB regimen has not yet been established. We compared the analgesic effects between a continuous ACB group and fentanyl-based intravenous patient-controlled analgesia (IV-PCA) with a single-shot ACB group.
Methods
Patients who underwent TKA were randomly allocated to either a continuous ACB group (Group CACB) or IV-PCA with a single-shot ACB group (Group IVACB). Before the surgery, ultrasound guided ACB with 0.5% ropivacaine 20 cc was provided to all patients. Before skin incision, the infusion system (0.2% ropivacaine through an adductor canal catheter in group CACB vs. intravenous fentanyl in group IVACB) was connected. The postoperative pain severity; the side effects of local anesthetics and opioids; administration of rescue analgesics and anti-emetics; and sensorimotor deficits were measured.
Results
Postoperative pain severity was significantly higher in the IVACB group at 30 min, 4 h, 24 h, and 48 h after surgery. The averages and standard deviations (SD) of the NRS score of postoperative pain were 0.14 ± 0.37, 4.57 ± 2.37, 6.00 ± 1.63, and 4.28 ± 1.49, respectively in the IVACB group. Rescue analgesic requirements and quadriceps muscle strength were not statistically different between the groups throughout the postoperative period. Moreover, rescue antiemetic requirements were higher in group IVACB than group CACB.
Conclusions
In this study, the continuous ACB provided superior analgesia and fewer side effects without any significant motor deficit than the IV-PCA with a single-shot ACB.
Keywords: Analgesia, Fentanyl, Knee replacement arthroplasty, Local anesthetics, Nausea, Opioid, Pain management, Patient controlled analgesia, Postoperative pain, Ropivacaine, Vomiting
INTRODUCTION
The prevalence of knee osteoarthritis is increasing worldwide due to the aging population. A standard surgical intervention for advanced knee osteoarthritis is total knee arthroplasty (TKA), and demand for this surgery is rising. TKA is known to be a painful orthopedic procedure and moderate to severe pain is common, especially immediately postoperatively and during active motion [1]. The proportion of patients complaining of chronic pain after TKA is as much as 34%, and the intensity of early postoperative pain is associated with increased chronic pain after TKA [2]. Therefore, postoperative pain management is of utmost importance for patient outcome and satisfaction, and many studies have reported that multimodal pain management was necessary [1,2,3].
The femoral nerve block (FNB) is used as a conventional postoperative analgesic method for patients undergoing TKA. However, the FNB may reduce the quadriceps muscle strength [4], which is, in turn, associated with an increased risk of falling, hospitalization, and impaired postoperative recovery [5].
An ultrasound technique, t he a dductor canal block (ACB), has been developed as an alternative to FNB. Many studies report that the analgesic effect of ACB is similar to that of FNB, but with a relative sparing of quadricep strength [6] and an increase in ambulatory ability (the Time-Up-and-Go test, 10 m walk, or 30-second Chair test) [7]. Moreover, the continuous ACB, compared with the continuous FNB, is associated with a seven-fold increase in early a mb ulation [8]. TKA a lone r educes q uadriceps muscle strength [9], and many patients undergoing TKA are female or over 65 years of age. Thus, TKA patients are already at a greater risk of falls [10], underscoring the clinical importance of preserving muscle strength, using methods such as the ACB. However, a comparative study of the single-shot versus continuous ACB is lacking. Although Shah et al. [11] compared postoperative analgesia, ambulation ability, and early functional recovery following a single-shot or continuous ACB, their study was conducted in a different population using a different technique.
This study sought to explore the postoperative analgesic efficacy of continuous compared to fentanyl based intravenous patient controlled analgesia (IV-PCA) with a single-shot ACB under general anesthesia for TKA.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of Chung-Ang University Hospital (C2016119 [1862]), and was registered by the Clinical Research Information Service (CRIS: KCT0002121). Written informed consent was obtained from all participants, and the study was carried out based on the principles of the Declaration of Helsinki (2000).
All patients at our institution with an American Society of Anesthesiologists physical status of 1 to 3, aged between 20 and 70 years, and undergoing TKA with general anesthesia at Chung-Ang University Hospital, were assessed for eligibility for inclusion in the study between July 2016 and March 2017. To minimize the effects of the surgical technique, only patients who had undergone TKA by a single team of surgeons were included. All surgeries were done by using a modified subvastus approach with a midline skin incision. Before suturing the skin incision site, the intra-articular infiltration (20 cc 0.2% ropivacaine + 500 mg ceftezole + 40 mg triamcinolone acetate, the latter not included in patients with diabetic mellitus) was done in all patients.
Patients with a history cardiovascular, respiratory, or psychiatric disease, or obesity (body mass index ≥ 35 kg/m2), a history of substance abuse, allergy to local anesthetic agents, and nerve block contraindications, including coagulopathy or bleeding tendency, chronic pain syndrome, or neuropathy affecting the lower limbs, were excluded. The investigator, who was not involved in the intervention or data collection, assessed patients for study eligibility.
The study was performed using a prospective randomized method. An investigator, with a degree in statistics (HK), used a random table generated using PASS 11 (NCSS, Kaysville, UT) to randomly assign all participants to one of t wo g roups: 1) the continuous a dductor canal b lock group (Group CACB; n = 22), or 2) the IV-PCA with a single-shot adductor canal block group (Group IVACB; n = 22). According to the group allocation, one pain specialist (MKK) with no further role in any other step of the study performed adductor canal blocks or catheterization for the continuous adductor canal block. These procedures were conducted 1 h before surgery. For minimizing bias, one investigator (HYM) was involved in data collection for group CACB, and a second investigator (WYS) was involved in data collection for the group IVACB. The collected data were coded in numerical form so that the group allocation could not be known, and a third investigator (HK) was asked to analyze the data.
1. Intervention
All patients' vital signs were monitored by electrocardiography, and for noninvasive blood pressure, heart rate, and peripheral oxygen saturation before and throughout the procedure.
For the adductor canal block or catheterization, patients were placed in a supine position, with their operative hip joint slightly externally rotated and knee joint flexed. Skin preparation was aseptically conducted using povidone iodide (Besetine solution; HyunDai Pharm. Co., Ltd., Seoul, South Korea) and chlorhexidine (Hexial solution [2%]; Huniz. Co., Ltd., Busan, South Korea).
The procedures were conducted with an ultrasound-guided technique using an ACUSON P300™ with a 4–13 MHz linear transducer (Siemens Medical Solutions, Malvern, PA). The transducer was placed on the mid-thigh, and the proximal and distal ends of the adductor canal were identified. The level at which the medial border of the sartorius muscle crossed the adductor longus muscle was identified as the proximal end of the adductor canal. The level at which the femoral artery separated from the sartorius muscle and passed through the adductor hiatus was identified as the distal end of the adductor canal [12]. The middle of these two points was considered the optimal needle insertion site, and skin infiltration with 2% mepivacaine (400 mg/20 ml; Emcaine; Reyon Pharm. Co., Ltd., Seoul, South Korea) was done.
A 17-gauge Tuhoy needle was advanced using an in-plane technique, from the lateral to medial side toward the saphenous nerve, located at the supero-lateral side of the femoral artery within the adductor canal. In group CACB, a 17-gauge single orifice flexible epidural catheter (EPINA PLUS; ACE Medical Co., Ltd., Seoul, South Korea) was inserted through the needle, and advanced cephalad 3 cm from the needle along the perineural structure. The location of the catheter tip was confirmed by injecting 5 ml of 0.5% ropivacaine (100 mg/ 20 ml; Rocaine®, Reyon Pharm., Co., Ltd., Seoul, South Korea) through the catheter. Subsequently, subcutaneous tunneling of the catheter was performed, as previously reported by Mariano et al. [13]. A total of 20 ml of 0.5% ropivacaine (100 mg/ 20 ml; Rocaine; Reyon Pharm., Co., Ltd., Seoul, South Korea) was injected into both groups (an additional 15 ml via an epidural catheter in group CACB, and 20 ml via a needle in group IVACB).
No patients received premedication before anesthesia. All patients underwent general anesthesia according to a standard protocol 30 min after the procedures. Both groups received the standard monitoring methods, which included electrocardiography, non-invasive blood pressure assessment, and pulse oximetry was initiated after the patients entered the operating room. First, 5 mg/kg sodium pentothal was administered intravenously, followed by 0.6-mg/kg rocuronium after confirmation of the patients' loss of consciousness. Thereafter, intubation was performed. Desflurane with oxygen/nitrous oxide in 0.5 FiO2 was administered at a 1.5–2 minimum alveolar concentration to maintain anesthesia, and the ETCO2 was maintained at 35–40 mmHg.
An identical computerized patient-controlled analgesia (PCA) system (Accumate 1100; WOO Young Medical Co., Ltd., Jincheon-Gun, South Korea) was used in both groups, and initiated at the time of increasing tourniquet pressure on the operative leg, namely just before the first skin incision. In group CACB, 0.2% ropivacaine (40 mg/ 20 ml; Rocainer®, Reyon Pharm. Co., Ltd., Seoul, South Korea) was infused through the catheter inserted in the adductor canal at a basal rate of 5 ml/h, with a lockout interval of 15 min, and a bolus of 5 ml.
In group IVACB, fentanyl (100 mcg/ 2 ml; fentanyl citrate injection; Daewon Pharm. Co., Ltd., Seoul, South Korea) was intravenously infused at 0.4 mcg/kg/h, with a lockout interval of 15 min, and a bolus of 0.4 mcg/kg (total volume including normal saline: 100 ml). One day prior to surgery, patients were instructed on how to use the PCA system and to press the bolus button as needed. No patients received intraoperative opioids, except the intervention mentioned above.
During the postoperative period, if patients complained of pain, nursing staff called the investigator (HJL). This investigator assessed the pain score, and injected 50 mg of tramadol (50 mg/1 ml; Zipan Inj.; Ilsung Pharm. Co., Ltd., Seoul, South Korea) as an additional analgesic when the numeric rating scale (NRS) pain score was 4 or greater. If the patient complained of persistent pain despite two repeated tramadol injections at 4-h intervals, or if the patient complained of severe pain (an NRS pain score of 8 or greater), 100 mcg of fentanyl was intravenously injected. Palonosetron (0.075 mg; Aloxi; Helsinn Brex Pharm. Co, Ltd, Dublin, Ireland) was injected twice a day, from postoperative day 0 to 3 on demand.
2. Studied variables
Post-operative pain in the operative lower limb was the primary outcome variable in this study. Pain severity was evaluated using an NRS (with 0 being no pain and 10 being the worst pain imaginable). Secondary outcome variables included the dosage converted to a morphine-equivalent of additional rescue analgesics, a muscle strength evaluation using the Medical Research Council scale [14], and vital signs (heart rate, systolic blood pressure, and diastolic blood pressure). Adverse effects such as nausea, vomiting, lightheadedness, and dizziness were also evaluated.
The pain NRS, vital signs, and adverse effects were evaluated pre-procedure, 30 min post-procedure, and 30 min, 2 h, 4 h, 8 h, 24 h, and 48 h after surgery. The use of rescue analgesics was evaluated at the same time points, except for pre-procedure and 30 min post-procedure. Muscle strength was estimated by the medical research council scale of quadriceps muscle strength one day before surgery and postoperatively on days 1, 2, 3, and 4.
3. Statistical analysis
To estimate the required sample size, a pilot study (group IVACB, n = 7) was conducted. The means (standard deviations, SD) for postoperative pain NRS scores at 30 min, 2 h, 4 h, 8 h, 24 h, and 48 h after surgery were 0.14 (0.37), 1.28 (0.95), 4.57 (2.37), 4.57 (2.29), 6.00 (1.63), and 4.28 (1.49), respectively. The autocorrelation between adjacent measurements of the same individual was 0.7. For the power calculation, we assumed that first-order autocorrelation adequately represented the autocorrelation pattern. We wanted to detect a 30% decrease in NRS scores in group CACB compared to group IVACB. We used PASS 11™ software (NCSS, Kaysville, UT) to calculate the sample size. Results showed that 22 subjects were needed per group with an α of 0.05 and a power of 80%.
For comparing both groups, the Shapiro-Wilk test was used to test for normality of variables. The normally distributed data including age, weight, BMI, anesthesia time, and operative time were presented as mean (SD) after analysis with parametric tests. The non-normally distributed data was presented as median (P25–P75) after analysis with nonparametric test. Because of Mauchly's sphericity test for the NRS scores of postoperative pain, rescue analgesics, heart rate, systolic blood pressure, diastolic blood pressure, and medical research council scale of muscle strength indicated that the assumption of sphericity had been violated (χ2 [27] = 142.9, P < 0.001, χ2 [14] = 149.2, P < 0.001, χ2 [27] = 90.5, P < 0.001, χ2 [27] = 142.3, P < 0.001, χ2 [27] = 69.6, P < 0.001 and χ2 [9] = 42.8, P < 0.001, respectively), we used Wilk's Lambda multivariate analysis of variance (MANOVA), followed by the t-test with the Bonferroni correction. Descriptive variables were analyzed using either the χ2-test or Fisher's exact test, as appropriate. Data are presented as mean (standard deviation, SD), or as an absolute number, and P values < 0.05 were considered statistically significant.
All of the statistical analyses were conducted using statistical software (IBM SPSS statistics version 23, IBM Corp., Armonk, NY).
RESULTS
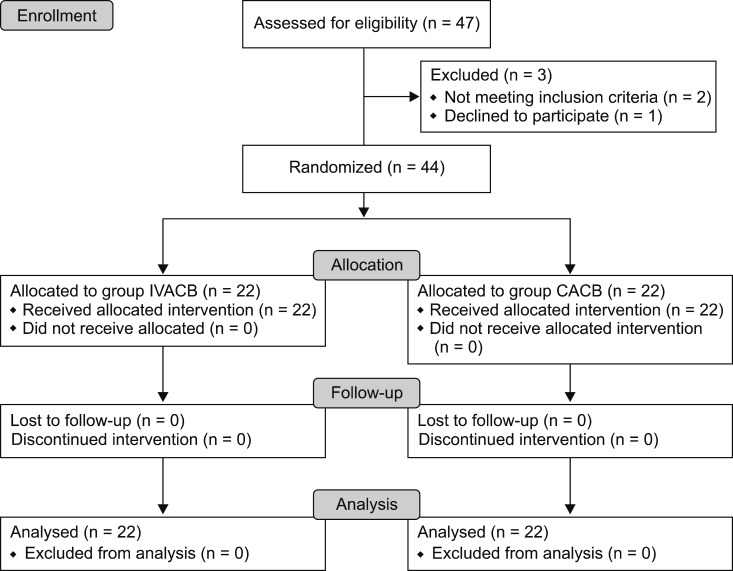
Forty-seven patients who u nderwent TKA b etween July 2016 and March 2017 were assessed for eligibility. Two patients did not meet the study criteria, and one refused participation. Forty-four patients were randomly assigned to either group CACB or group IVACB (Fig. 1).
Fig. 1. CONSORT flow diagram.
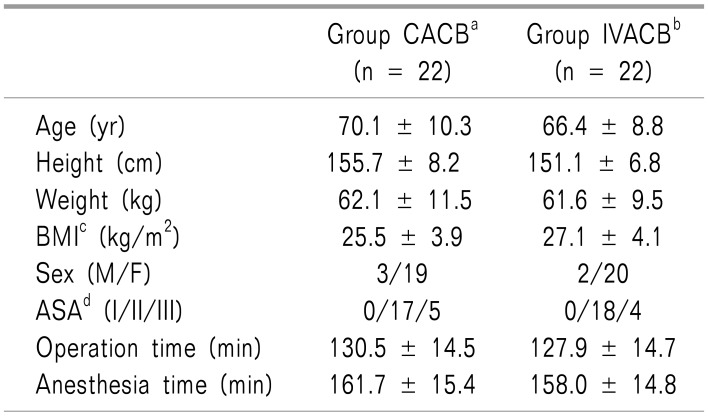
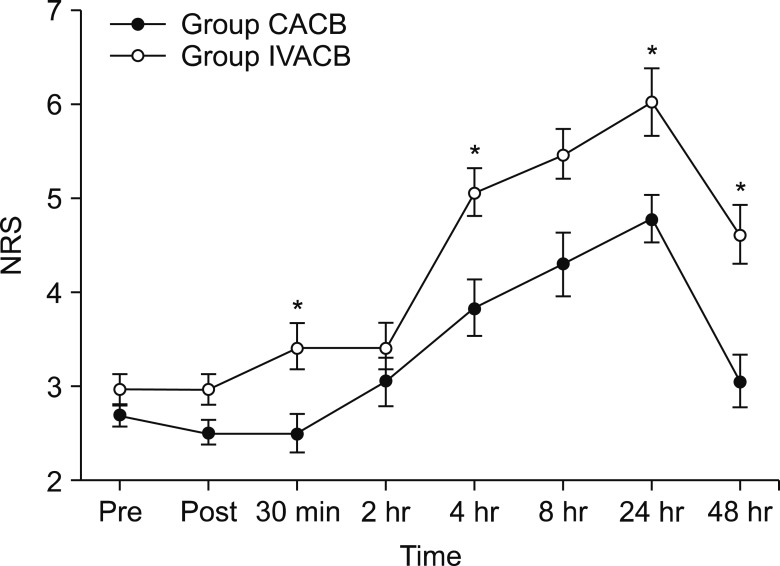
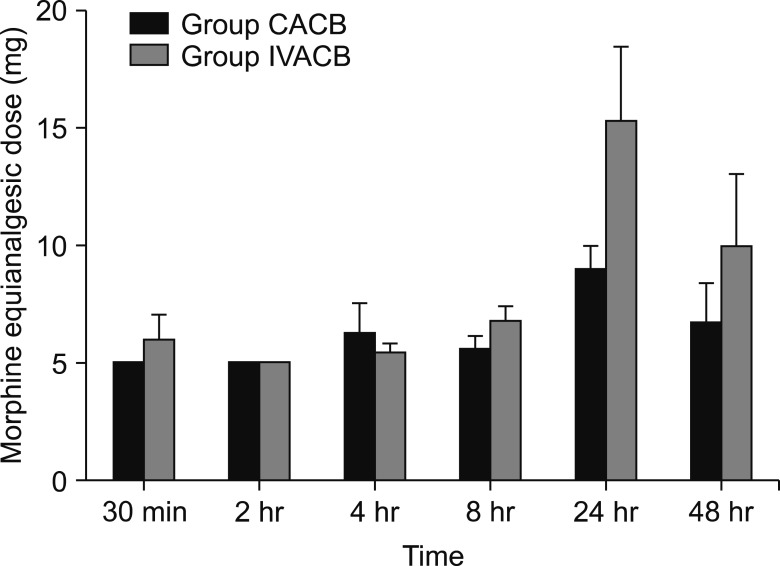
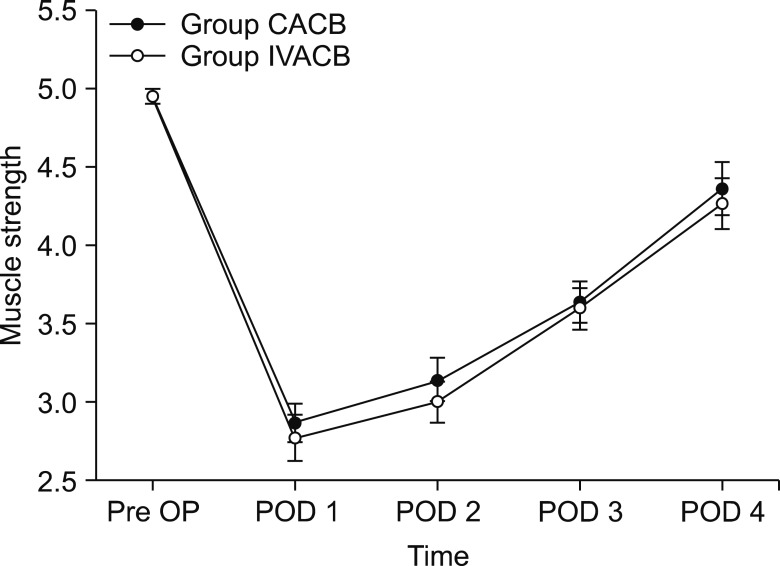
The demographic data were not significant different between the groups, except for height (Table 1). The parameters about vital signs also showed no difference. The NRS score of postoperative pain was significantly different at the time points of 30 min, 4 h, 24 h, and 48 h after surgery (Fig. 2). The morphine equi-analgesic dose of additional rescue analgesics was not significantly different at all-time points (P = 0.142; Fig. 3). However, the morphine equi-analgesic doses of total analgesics were significantly different (mean ± SD: 10.5 ± 8.7 in group CACB vs. 23.8 ± 22.9 in group IVACB, P = 0.016). The medical research council scale for estimating the muscle strength of the quadriceps muscle on the operative lower limb showed no significant difference between the groups (P = 0.990; Fig. 4).
Table 1. Demographic Data.
Values are expressed as means ± standard deviation, or as absolute numbers. aContinuous adductor canal block, bIntravenous patient-controlled analgesia with adductor canal block, cBody mass index, dAmerican Society of Anesthesiologists.
Fig. 2. Changes in numeric rating scale perioperative pain severity. NRS: numeric rating scale, CACB: continuous adductor canal block, IVACB: intravenous patient-controlled analgesia with adductor canal block, Pre: before ACB, Post: 30 minutes after ACB, 30 min: 30 minutes after surgery, 2 h: 2 hours after surgery, 4 h: 4 hours after surgery, 8 h: 8 hours after surgery, 24 h: 24 hours after surgery, 48 h: 48 hours after surgery. *P < 0.05 compared with Group IVACB.
Fig. 3. Morphine equi-analgesic dose of rescue analgesics. CACB: continuous adductor canal block, IVACB: intravenous patient-controlled analgesia with adductor canal block, 30 min: 30 minutes after surgery, 2 h: 2 hours after surgery, 4 h: 4 hours after surgery, 8 h: 8 hours after surgery, 24 h: 24 hours after surgery, 48 h: 48 hours after surgery. Morphine equi-analgesic dose of each time points were the summed dosage from the end of last measurement up to the current measurement.
Fig. 4. Medical Research Council scale of quadriceps muscle strength. CACB: continuous adductor canal block, IVACB: intravenous patient-controlled analgesia with adductor canal block, Pre-op: 1 day before surgery, POD 1: postoperative day 1, POD 2: postoperative day 2, POD 3: postoperative day 3, POD 4: postoperative day 4.
None of the patients showed any adverse effects due to improper injection of local anesthetics, such as nausea, vomiting, lightheadedness, or dizziness. Rescue antiemetic was significantly different between the groups [0.0 (0.0–0.0) in group CACB vs. 0.0 (0.0–1.0) in group IVACB, P = 0.046].
DISCUSSION
There were significant differences in the NRS scores between the groups at 30 min, 4 h, 24 h, and 48 h after surgery. The morphine equi-analgesic doses of total rescue analgesics were significantly different between the groups, b ut q uadriceps muscle strengths were n ot significantly different.
Postoperative pain after TKA is known to range from moderate (30% of patients) to severe (60% of patients) [15]. Improper management for postoperative pain may induce various immobility related complications, such as deep venous thrombosis, pulmonary embolisms, muscle weakness, and chronic pain. Therefore, post-TKA analgesia is crucial, not only for patients' satisfaction, but for improving surgical outcomes and reducing complications. IV-PCA, epidural analgesia, lumbar plexus blocks, and FNBs are used as analgesic methods [1].
When postoperative pain is managed by a single analgesic method, IV-PCA requires substantial opioids, and epidural analgesia has a greater risk of hypotension or urinary retention [16]. Thus, previous studies recommend multimodal analgesic methods [1,2,3]. There is a particular interest in multimodal analgesia using a peripheral nerve block, due to lower complication rates than with a central nerve block, and a number of studies have sought to compare their effectiveness.
FNB is commonly used for postoperative analgesia after TKA. However, given its various motor innervations to the quadriceps, sartorius, and pectineus muscles, the femoral nerve is responsible for impeding early rehabilitation, prolonging hospital stays, and increasing the risk of falling after the FNB [17]. Thus, practitioners have focused their attentions on the ACB, which leads to less quadriceps muscle weakness than the FNB [4].
The adductor canal is a triangular shaped intermuscular space containing the neurovascular structure of the thigh, from the apex of the femoral triangle to the end of the adductor hiatus [12]. Local anesthetics injected into the adductor canal provide a blockade not only to the saphenous nerve, but also to the vastus medialis nerve, medial femoral cutaneous nerve, articular branches of the obturator nerve, and medial retinacular nerve [18].
There is still considerable controversy about the analgesic effects of the ACB, which have been reported to be better than those of the FNB [19], and similar [8], or inferior [20] in others. Recently, two systematic reviews and meta-analyses exploring the effectiveness of the ACB compared to the FNB concluded that the ACB resulted in better functional recovery and similar analgesic effects.
To maximize the desirable analgesia effects of the ACB whilst also minimizing undesirable motor weaknesses, it is important to understand the nerve pathway in the adductor canal and knee innervation. An anatomic study [12] reported that the vastus medialis nerve showed a high degree of innervation to the knee joint capsule and the saphenous nerve showed relatively modest innervation, but the obturator nerve played a small and largely inconsistent role. Unlike previous studies, suggesting a distal injection in the adductor canal for the prevention of motor blockade [4], their study offered new insight into the injection site of the ACB. Thus, we injected the ACB into the midportion of the canal, not the mid-thigh, to block both the saphenous and vastus medialis nerves, leaving the distal third of the canal and branch to supply innervation to the knee joint capsule [12].
Although the sensory supply of the knee is innervated by a branch of the femoral and sciatic nerves, neither the trajectory of the sciatic nerve or its branches pass through the adductor canal [21]. However, the adductor canal is not a closed space that communicates with the proximal end of the femoral triangle or the distal end of the adductor hiatus [22]. The injected local anesthetics in the adductor canal spread to the femoral triangle [21,23], even as far as the popliteal fossa [24]. Thus, the degree of pain relief and muscle weakness might be influenced by these characteristics of the ACB.
Our results showed that postoperative pain severity in the NRS was significantly higher in group IVACB than group CACB at 30 min, 4 h, 24 h, and 48 h after surgery. The NRS scores showed a steady upwards curve in both groups until 24 h after surgery. This result was similar to a previous systematic review [1]. Although there were no statistically significant differences at any time point, the morphine equi-analgesic dose of the rescue analgesics tended to be greater in group IVACB than group CACB. In addition, the morphine equi-analgesic dose of total rescue analgesics showed a significant difference.
Shah et al. [11] also compared the analgesic effect of the continuous ACB and the single-shot ACB. Unlike our study, which used general anesthesia, they used spinal anesthesia for surgery and thus the analgesic effects of the ACB may have been overestimated. Nevertheless, in comb ination with our f indings, i t is l ikely that the analgesic effect of the continuous ACB is superior to that of the single-shot ACB. As the single-shot ACB might not last over 24 h [25], and pain relief after 24 h post-surgery may be a true testament to the efficacy of IV-PCA. In our study, there was a significant between-group difference in the NRS scores after 24 h of surgery, indicating that the analgesic effect of the continuous ACB is more effective than IV-PCA.
Our result in quadriceps muscle strength supports those of a previous study [11]; ambulation ability and early functional recovery were not significantly different between continuous and single-shot ACB. Quadriceps muscle strength maximally decreased 24 h after surgery, and then gradually recovered over 4 days postoperatively in b oth groups. No significant differences between the groups were recorded for quadriceps muscle strength at any time point.
The reasons for the decrease in muscle strength may be multiple. One probable factor is a motor blockade by the proximal spread, volume, and concentration of local anesthetics. Although several previous studies report that the ACB could reduce quadriceps muscle strength [12,23] most studies convincingly report that the ACB preserves quadriceps muscle strength better than other peripheral nerve blocks used for postoperative analgesia after TKA [6,7,8]. In our study, quadriceps muscle strength diminished even after the analgesic effect of the single-shot ACB had worn off, suggesting that other factors were related to motor weakness other than the motor block by the ACB. TKA, per se, greatly reduces muscle strength [9], and postoperative pain, swelling, and reflex inhibition could also result in such consequences [26]. Given that quadriceps muscle weakness is related to increased incidence of falling [1], close observation of patients receiving TKA is necessary for fall prevention, regardless of the postoperative analgesic method.
Although the ACB has advantages over conventional analgesic methods, there are several side effects like other peripheral nerve blocks, such as catheter site infection [27], nerve palsy [28], heel ulceration due to a decrease of sensation [29], and a risk of falls due to motor blockade. In addition, the use of a catheter comes at an additional financial cost and requires specialist labor for catheter management [30]. Fortunately, in our study, there were no side-effects of the nerve block and local anesthetic use, but practitioners must always consider the risk-outcome benefits when using a peripheral nerve bock for multimodal analgesia.
Our study has several limitations. Firstly, double-blinding was not conducted. For double-blinding, patients in group CACB would need to carry an additional PCA machine connected to the intravenous sham line, and patients in group IVACB would require an adductor canal sham catheter. We believed that such infusion machines would disturb patient rehabilitation, and increase the incidence of falling. Nevertheless, this may be a confounding factor for our results. Secondly, we assessed mean postoperative pain using the NRS alone. The articular branch of the common peroneal nerve also supplies the knee joint. To determine whether the analgesic effect was influenced by spreading local anesthetics to the popliteal fossa, further assessment of pain severity in each anatomical area innervated by the saphenous, vastus medialis, obturator, and peroneal nerves is required. Finally, we used IV-PCA in group IVACB, and omitted the single-shot ACB only group. We thought that a single-shot ACB without IV-PCA was unethical, given the severe postoperative pain that patients could feel after nerve block washout.
In conclusion, continuous ACB is an excellent alternative analgesic method to intravenous fentanyl infusion with a single-shot ACB after TKA, as it minimizes the use of additional analgesics and antiemetics. Thus, further work is required to explore the analgesic effectiveness, in terms of local anesthesia volume and concentration, as well as the combined application of additional nerve blocks.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
References
- 1.Fischer HB, Simanski CJ, Sharp C, Bonnet F, Camu F, Neugebauer EA, et al. A procedure-specific systematic review and consensus recommend ations for postoperative analgesia following total knee arthroplasty. Anaesthesia. 2008;63:1105–1123. doi: 10.1111/j.1365-2044.2008.05565.x. [DOI] [PubMed] [Google Scholar]
- 2.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2:e000435. doi: 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung MY, Kim CJ. The effect of bilateral femoral nerve block combined with intravenous patient-controlled analgesia after a bilateral total knee replacement. Korean J Pain. 2008;21:211–216. [Google Scholar]
- 4.Jæger P, Zaric D, Fomsgaard JS, Hilsted KL, Bjerregaard J, Gyrn J, et al. Add uctor canal block versus femoral nerve block for analgesia after total knee arthroplasty: a randomized, double-blind study. Reg Anesth Pain Med. 2013;38:526–532. doi: 10.1097/AAP.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 5.Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111:1552–1554. doi: 10.1213/ANE.0b013e3181fb9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DH, Lin Y, Goytizolo EA, Kahn RL, Maalouf DB, Manohar A, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120:540–550. doi: 10.1097/ALN.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 7.Shah NA, Jain NP. Is continuous adductor canal block better than continuous femoral nerve block after total knee arthroplasty? Effect on ambulation ability, early functional recovery and pain control: a randomized controlled trial. J Arthroplasty. 2014;29:2224–2229. doi: 10.1016/j.arth.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Mudumbai SC, Ganaway T, Kim TE, Howard SK, Giori NJ, Shum C, et al. Can bedside patient-reported numbness predict postoperative ambulation ability for total knee arthroplasty patients with nerve block catheters? Korean J Anesthesiol. 2016;69:32–36. doi: 10.4097/kjae.2016.69.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87:1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackerman DB, Trousdale RT, Bieber P, Henely J, Pagnano MW, Berry DJ. Postoperative patient falls on an orthopedic inpatient unit. J Arthroplasty. 2010;25:10–14. doi: 10.1016/j.arth.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Shah NA, Jain NP, Panchal KA. Adductor canal blockade following total knee arthroplasty-continuous or single shot technique? Role in postoperative analgesia, ambulation ability and early functional recovery: a randomized controlled trial. J Arthroplasty. 2015;30:1476–1481. doi: 10.1016/j.arth.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Burckett-St Laurant D, Peng P, Girón Arango L, Niazi AU, Chan VW, Agur A, et al. The nerves of the adductor canal and the innervation of the knee: an anatomic study. Reg Anesth Pain Med. 2016;41:321–327. doi: 10.1097/AAP.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 13.Mariano ER, Kim TE, Wagner MJ, Funck N, Harrison TK, Walters T, et al. A randomized comparison of proximal and distal ultrasound-guided adductor canal catheter insertion sites for knee arthroplasty. J Ultrasound Med. 2014;33:1653–1662. doi: 10.7863/ultra.33.9.1653. [DOI] [PubMed] [Google Scholar]
- 14.Compston A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty's Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O'Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain. 2010;133:2838–2844. doi: 10.1093/brain/awq270. [DOI] [PubMed] [Google Scholar]
- 15.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Fowler SJ, Symons J, Sabato S, Myles PS. Epidural analgesia compared with peripheral nerve blockade after major knee surgery: a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2008;100:154–164. doi: 10.1093/bja/aem373. [DOI] [PubMed] [Google Scholar]
- 17.Kandasami M, Kinninmonth AW, Sarungi M, Baines J, Scott NB. Femoral nerve block for total knee replacement-a word of caution. Knee. 2009;16:98–100. doi: 10.1016/j.knee.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Manickam B, Perlas A, Duggan E, Brull R, Chan VW, Ramlogan R. Feasibility and efficacy of ultrasound-guided block of the saphenous nerve in the adductor canal. Reg Anesth Pain Med. 2009;34:578–580. doi: 10.1097/aap.0b013e3181bfbf84. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40:925–933. doi: 10.1007/s00264-015-2998-x. [DOI] [PubMed] [Google Scholar]
- 20.Memtsoudis SG, Yoo D, Stundner O, Danninger T, Ma Y, Poultsides L, et al. Subsartorial adductor canal vs femoral nerve block for analgesia after total knee replacement. Int Orthop. 2015;39:673–680. doi: 10.1007/s00264-014-2527-3. [DOI] [PubMed] [Google Scholar]
- 21.Deloach JK, Boezaart AP. Is an adductor canal block simply an indirect femoral nerve block? Anesthesiology. 2014;121:1349–1350. doi: 10.1097/ALN.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 22.Ishiguro S, Yokochi A, Yoshioka K, Asano N, Deguchi A, Iwasaki Y, et al. Technical communication: anatomy and clinical implications of ultrasound-guided selective femoral nerve block. Anesth Analg. 2012;115:1467–1470. doi: 10.1213/ANE.0b013e31826af956. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Lesser JB, Hadzic A, Reiss W, Resta-Flarer F. Adductor canal block can result in motor block of the quadriceps muscle. Reg Anesth Pain Med. 2014;39:170–171. doi: 10.1097/AAP.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 24.Gautier PE, Hadzic A, Lecoq JP, Brichant JF, Kuroda MM, Vandepitte C. Distribution of injectate and sensory-motor blockade after adductor canal block. Anesth Analg. 2016;122:279–282. doi: 10.1213/ANE.0000000000001025. [DOI] [PubMed] [Google Scholar]
- 25.Joshi G, Gandhi K, Shah N, Gadsden J, Corman SL. Peripheral nerve blocks in the management of postoperative pain: challenges and opportunities. J Clin Anesth. 2016;35:524–529. doi: 10.1016/j.jclinane.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 26.Holm B, Kristensen MT, Bencke J, Husted H, Kehlet H, Bandholm T. Loss of knee-extension strength is related to knee swelling after total knee arthroplasty. Arch Phys Med Rehabil. 2010;91:1770–1776. doi: 10.1016/j.apmr.2010.07.229. [DOI] [PubMed] [Google Scholar]
- 27.Cuvillon P, Ripart J, Lalourcey L, Veyrat E, L'Hermite J, Boisson C, et al. The continuous femoral nerve block catheter for postoperative analgesia: bacterial colonization, infectious rate and adverse effects. Anesth Analg. 2001;93:1045–1049. doi: 10.1097/00000539-200110000-00050. [DOI] [PubMed] [Google Scholar]
- 28.Feibel RJ, Dervin GF, Kim PR, Beaulé PE. Major complications associated with femoral nerve catheters for knee arthroplasty: a word of caution. J Arthroplasty. 2009;24:132–137. doi: 10.1016/j.arth.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Edwards JL, Pandit H, Popat MT. Perioperative analgesia: a factor in the development of heel pressure ulcers? Br J Nurs. 2006;15:S20–S25. doi: 10.12968/bjon.2006.15.Sup1.20688. [DOI] [PubMed] [Google Scholar]
- 30.Ludwigson JL, Tillmans SD, Galgon RE, Chambers TA, Heiner JP, Schroeder KM. A comparison of single shot adductor canal block versus femoral nerve catheter for total knee arthroplasty. J Arthroplasty. 2015;30:68–71. doi: 10.1016/j.arth.2015.03.044. [DOI] [PubMed] [Google Scholar]