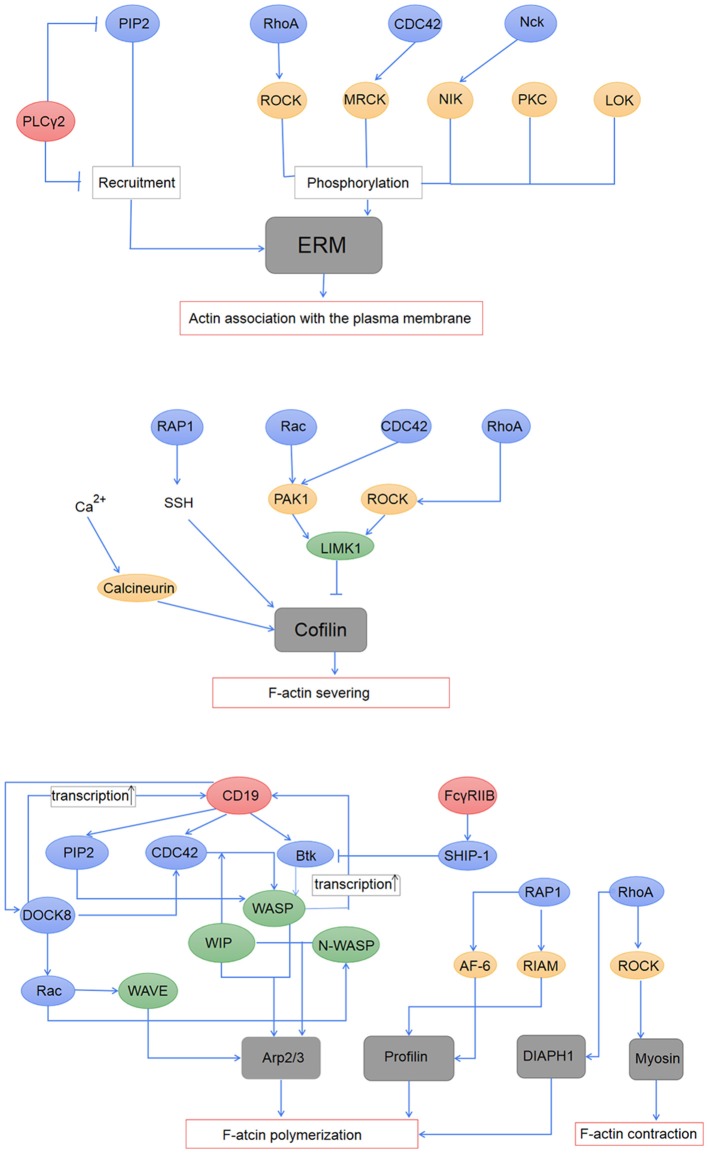
Figure 2.
Regulation of BCR signaling on the actin cytoskeleton. The association of the actin cytoskeleton with the plasma membrane is mediated by activated ERM proteins. The ERM proteins are first recruited to the plasma membrane by PIP2, and then phosphorylated by PKC, LOK, and effector proteins of RhoA, CDC42, and Nck. PLCγ2 induced inactivation of the ERM proteins through its down-regulation on PIP2. Activation of cofilin induces F-actin severing, which is regulated by the Rho family and Rap1 GTPase, and also intracellular calcium. BCR signaling regulates actin polymerization mainly through the actin-nucleation promotion factor WASP and WAVE, both of which can promote the nucleation effect of Arp2/3. Profilin and DIAPH1, which are regulated by RAP1 and RhoA, respectively, are suggested to participate in actin polymerization during B-cell activation. BCR signaling also influences contraction of the actin cytoskeleton through the regulation of RhoA on myosin.