Abstract
Objective
This study aimed to evaluate the prognostic impact of age at diagnosis, and pretreatment hematologic markers, including lymphocyte percentage and the neutrophil-to-lymphocyte ratio (NLR), in patients with locally advanced cervical cancer (LACC) treated with definitive radiotherapy (RT).
Methods
A total of 392 patients with LACC (stage IIb to IVa) treated with cisplatin-based concurrent chemoradiotherapy or RT alone between 2001 and 2012 were retrospectively enrolled. Clinical data and pretreatment complete blood counts were extracted from electronic medical records of the patients, and analyzed. Treatment outcomes, progression-free survival (PFS), and overall survival (OS) were evaluated.
Results
Low lymphocyte percentage and a high NLR were associated with younger age, advanced stage, larger tumor size, lymph nodes metastasis, and treatment failure. The cut-off value for lymphocyte percentage and NLR was determined using a receiver operating characteristic curve. In univariate analysis, low lymphocyte percentage (≤24%) was associated with poor PFS and OS, while high NLR (>2.8) was significantly associated only with PFS. In multivariate analysis, both lymphocyte percentage (hazard ratio [HR], 0.59; 95% confidence interval [CI], 0.40–0.85; P=0.005) and NLR (HR, 1.55; 95% CI, 1.07–2.25; P=0.022) had independent prognostic value for PFS. Compared to younger patients (age ≤50 years), older patients (age >60 years) had a lower risk of death.
Conclusion
Although the lymphocyte percentage did not remain significant in multivariate analysis for OS, it was predictive of PFS and OS. Thus, lymphocyte percentage is a simple hematologic parameter with a significant prognostic value in patients with LACC treated with definitive RT.
Keywords: Hematologic test, Lymphocytes, Cervical cancer, Radiotherapy, Aging, Prognosis
Introduction
The incidence and mortality of cervical cancer have steadily decreased since cytological screening with the Papanicolaou (Pap) test was introduced. However, cervical cancer is still the most common gynecological cancer, with 3,500 new cases detected in 2014 [1]. It has been reported that only half of the target population participates in the Korean nationwide cervical cancer screening program [2]. Early-stage cervical cancer can be successfully treated by surgery. However, most cervical cancers develop in women who do not undergo regular screening for cervical cancer, and an advanced-stage tumor is commonly observed in such women.
The standard treatment for patients with locally advanced cervical cancer (LACC) is concurrent chemoradiotherapy (CCRT) with cisplatin [3]. Although CCRT reduces the rates of local and distant recurrence and improves disease-free survival, the recurrence rate is still high in patients with very large or advanced-stage tumors. In contrast to early-stage cervical cancer, which is usually treated by radical hysterectomy, there is a lack of pathologic parameters in patients with LACC who are treated with definitive radiotherapy (RT). Establishment of reliable prognostic or predictive markers is necessary for identification of high-risk patients who may benefit from adjuvant therapy.
Cancer outcomes are not only determined by tumor characteristics but are also influenced by patient-related factors [4]. A variety of host-related features have been implicated as prognostic factors for cancer-related survival including age, weight loss, performance status, and immune response. According to immunosurveillance theory, lymphocytes operate as guards against cancer by identifying and destroying malignant cells [5]. Compared to healthy women, those with cervical cancer have lower peripheral lymphocyte counts [6].
Both young age and low pretreatment lymphocyte counts have been reported to be associated with poor prognosis in patients with cervical cancer. A recent epidemiologic study in Korea revealed increasing incidence and mortality rates of cervical cancer in the younger population [7]. Pretreatment lymphocyte count has been shown to independently predict treatment outcomes in patients with LACC who are treated using RT or CCRT [8,9]. Pretreatment leukocytosis and neutrophilia also have been demonstrated to have an independent prognostic impact in these patients. A combination of these hematological parameters that represent the systemic inflammatory response, such as the neutrophil-lymphocyte ratio (NLR), has been demonstrated to have substantial prognostic value in cervical cancer [10,11,12,13,14,15,16,17,18]. Although the prognostic role of NLR has been confirmed by several studies, it has not yet been incorporated into clinical practice. Another hematologic marker, the lymphocyte-to-white blood cell (WBC) ratio (lymphocyte percentage), was also found to have prognostic value in nasopharyngeal carcinoma, hepatocellular carcinoma, and colorectal carcinoma [19,20,21]. However, to the best of our knowledge, no studies have investigated the prognostic value of lymphocyte percentage in patients with cervical cancer who undergo definitive RT or CCRT.
In order to identify more useful clinical parameters that predict RT response and prognosis for patients undergoing definitive RT, we investigated the prognostic impact of various hematologic markers including lymphocyte percentage and NLR in patients with LACC who were treated with definitive RT.
Materials and methods
1. Patients
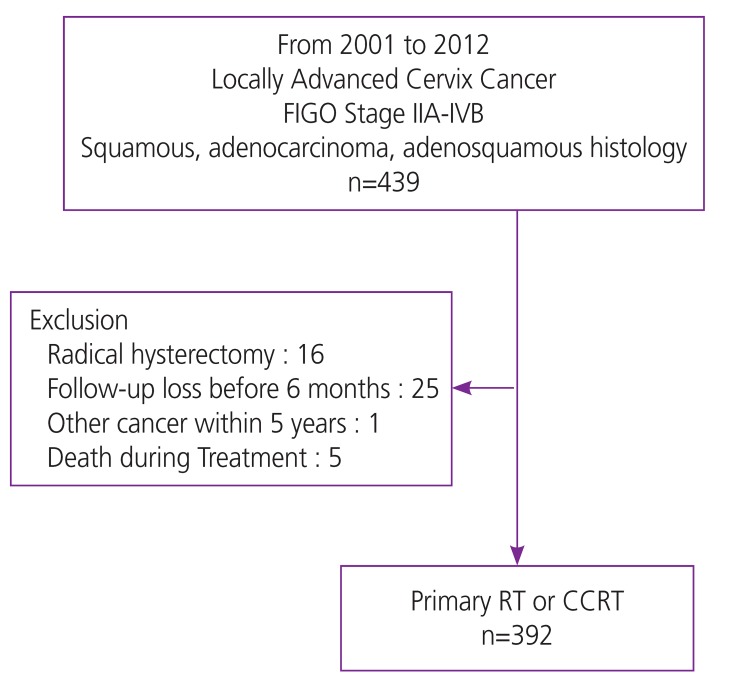
This study retrospectively enrolled 392 patients with cervical cancer classified as International Federation of Gynecology and Obstetrics (FIGO) stage IIb to IVa who were treated using primary RT or CCRT between 2001 and 2012 at Korea Cancer Center in Seoul, Korea. This patient cohort is described in Fig. 1. Clinicopathologic variables such as age, histological type, stage, tumor size, and primary treatment method were ascertained using the cancer registry and patient medical records, with permission from the Institutional Review Board. Patients with recurrent cervical cancer; those undergoing treatment with radical hysterectomy; those with histological cancer subtypes other than squamous cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma; those with concurrent hematologic or infectious disease; and those without complete blood cell count data with differential cell counts within 2 weeks of initiation of RT were excluded from the study. To analyze the association between age and hematologic markers, and the impact of age on the treatment outcomes for cervical cancer, patients were divided into three groups: young age group (age ≤50 years, n=115), intermediate age group (age 51–60 years, n=119), and old age group (age ≥61 years, n=158).
Fig. 1. Patient cohort description.
FIGO, International Federation of Gynecology and Obstetrics; RT, radiotherapy; CCRT, concurrent chemoradiotherapy.
Cervical cancer was staged according to the FIGO staging system using various examinations, including magnetic resonance (MR) imaging and abdominopelvic computed tomography (CT). Tumor size was determined by measuring the largest tumor diameter in 3-dimensional MR or CT images. Lymph node (LN) metastasis was defined as any pelvic or para-aortic LN with a diameter greater than 1 cm along the short axis on MR or CT images.
WBC, absolute neutrophil count (ANC), and absolute lymphocyte count (ALC) were obtained from complete blood cell count data at diagnosis. The lymphocyte percentage was calculated as the proportion of the ALC in the total WBC count. NLR was defined as the ANC divided by the ALC.
2. Treatment
All patients underwent either cisplatin-based CCRT or RT alone. Although CCRT was recommended to all patients as a standard therapy, some patients received RT only owing to various reasons including impaired renal function, poor performance status, and financial problems. The radiation protocol was as previously described [22]. The cisplatin-based CCRT regimens included weekly cisplatin (40 mg/m2), tri-weekly cisplatin (75 mg/m2 every 3 weeks), FP (500 mg/m2 5-fluorouracil + 50 mg/m2 cisplatin), or CP (500 mg/m2 cyclophosphamide + 50 mg/m2 cisplatin).
3. Follow-up
Patients were followed by physical examinations and Pap smears every 3 months for 2 years, and then every 6 months for the next 3 years. Chest radiography and imaging studies, such as pelvic CT, positron emission tomography (PET), or PET/CT imaging, were annually performed. Complete remission (CR) was defined as no evidence of disease at 6 months after diagnosis. Residual tumor or the appearance of a new lesion during the follow-up period was regarded as persistent disease (PD).
Recurrence was defined as the appearance of clinical, radiologic, or histologic evidence of disease after completion of CCRT or RT. Progression-free survival (PFS) was defined as the time from initial treatment to relapse noted on images or in histologic examinations, or the time to the final follow-up visit. OS was defined as the period from initial treatment to the final follow-up visit or the date of death, the data regarding which were obtained from the National Statistical Office.
4. Statistical analysis
The characteristics of different patient age groups were compared using χ2 test or Fisher's exact test. Student's t-test and analysis of variance were used to compare hematologic markers. Continuous variables including hematologic variables and tumor size were categorized using the median as the cut-off value. For NLR and lymphocyte percentage, receiver operating characteristic (ROC) curve analysis was used to determine the cut-off values. The Kaplan-Meier method, and log-rank tests and the Cox regression model were used for survival analysis. Statistical analyses were performed using SPSS for Windows, version 18 (SPSS, Inc., Chicago, IL, USA). Statistical significance was defined as P<0.05.
Results
1. Baseline patient characteristics
Baseline patient characteristics are presented in Table 1. The median age, tumor size, lymphocyte percentage, and NLR were 57 years, 50 mm, 24%, and 2.7, respectively. CCRT was administrated in 294 patients (75.0%). After completion of RT or CCRT, 39 patients (9.9%) were found to have PD. Clinicopathologic characteristics significantly differed between the age groups (Table 2). Patients in the young age group tended to have earlier-stage disease; however, these patients had larger tumor size and a higher rate of LN metastasis. The rate of undergoing CCRT decreased with increasing age (89.6%, 85.7%, and 56.3% in young, intermediate, and old age groups, respectively). Although patients in the young age group had earlier-stage diseases and high rate of CCRT, CR rate was the lowest in these patients.
Table 1. Baseline characteristics.
| Characteristics | Value (n=392) | |
|---|---|---|
| Age (yr) | 57 (28–88) | |
| Follow-up duration (mon) | 63.4 (4.4–165.1) | |
| WBC count (n/µL) | 7,680 (2,050–17,260) | |
| Hemoglobin (g/dL) | 11.7 (3.7–15.1) | |
| Neutrophil count (n/µL) | 5,110 (1,080–14,900) | |
| Neutrophil percentage | 65 (35–95) | |
| Lymphocyte count (n/µL) | 1,870 (490–4,640) | |
| Lymphocyte percentage | 24 (4–54) | |
| NLR | 2.70 (0.65–25.31) | |
| Tumor size (mm) | 50 (10–100) | |
| FIGO stage | ||
| IIB | 266 (67.9) | |
| IIIA | 13 (3.3) | |
| IIIB | 88 (22.4) | |
| IVA | 25 (6.4) | |
| Histology | ||
| SCC | 366 (93.4) | |
| Non-SCC | 24 (6.6) | |
| LN metastasis | ||
| No | 158 (40.3) | |
| Yes | 234 (59.7) | |
| Treatment | ||
| CCRT | 294 (75.0) | |
| RT only | 98 (25.0) | |
| Treatment result | ||
| CR | 353 (90.1) | |
| PD | 39 (9.9) | |
Data are presented as median (range) or number (%). NLR, neutrophil-to-lymphocyte ratio; FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; LN, lymph node; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; CR, complete remission; PD, persistent disease.
Table 2. Clinicopathologic differences based on age at diagnosis.
| Characteristics | Age groups | P-value | |||
|---|---|---|---|---|---|
| ≤50 (n=115) | 51–60 (n=119) | ≥61 (n=158) | |||
| FIGO stage | 0.015 | ||||
| IIB | 88 (76.5) | 70 (58.8) | 108 (68.4) | ||
| IIIA, IIIB, IVA | 27 (23.5) | 49 (41.2) | 50 (31.6) | ||
| Tumor size (mm) | 0.008 | ||||
| ≤50 | 57 (49.6) | 63 (52.9) | 105 (66.9) | ||
| >50 | 58 (50.4) | 56 (47.1) | 52 (33.1) | ||
| Histology | 0.820 | ||||
| SCC | 106 (92.2) | 112 (94.1) | 148 (93.7) | ||
| Others | 9 (7.8) | 7 (5.9) | 10 (6.3) | ||
| LN metastasis | <0.001 | ||||
| No | 31 (27.0) | 38 (31.9) | 89 (56.3) | ||
| Yes | 84 (73.0) | 81 (68.1) | 69 (43.7) | ||
| Treatment | <0.001 | ||||
| CCRT | 103 (89.6) | 102 (85.7) | 89 (56.3) | ||
| RT only | 12 (10.4) | 17 (14.3) | 69 (43.7) | ||
| Treatment result | 0.017 | ||||
| CR | 96 (83.5) | 109 (91.6) | 148 (93.7) | ||
| PD | 19 (16.5) | 10 (8.4) | 10 (6.3) | ||
Data are presented as number (%).
FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; LN, lymph node; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; CR, complete remission; PD, persistent disease.
2. Associations between hematologic variables and clinicopathologic factors
WBC, ANC, neutrophil percentage, ALC, lymphocyte percentage, and NLR differed according to clinical characteristics of the patients (Table 3). ANC was higher in patients who were younger, and had a more advanced-stage disease, bulky tumors, or LN metastasis. In contrast, ALC was higher in patients who were older and had no LN metastasis. Both lymphocyte percentage and NLR had a significant association with age, stage, tumor size, and LN metastasis. All six hematologic variables were predictive of treatment response.
Table 3. Differences of hematologic variables according to clinicopathologic characteristics.
| Characteristics | WBC | Neutrophil | Lymphocyte | NLR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute count | Absolute count | Percentage | Absolute count | Percentage | |||||||||
| Mean±SD | P-value | Mean±SD | P-value | Mean±SD | P-value | Mean±SD | P-value | Mean±SD | P-value | Mean±SD | P-value | ||
| Age group | 0.027 | 0.013 | 0.011 | <0.001 | 0.002 | 0.002 | |||||||
| ≤50 | 8,601±2,998 | 5,931±2,823 | 66.7±11.6 | 1,887±686 | 23.9±10.0 | 3.71±3.04 | |||||||
| 51–60 | 7,598±2,621 | 5,122±2,260 | 65.6±9.9 | 1,768±570 | 24.9±8.7 | 3.11±1.53 | |||||||
| >60 | 7,983±2,668 | 5,135±2,259 | 62.9±11.0 | 2,109±760 | 27.7±9.5 | 2.78±1.73 | |||||||
| FIGO stage | 0.005 | <0.001 | 0.002 | 0.146 | <0.001 | 0.016 | |||||||
| IIB | 7,698±2,429 | 5,011±2,123 | 63.6±11.0 | 1,976±737 | 27.0±9.6 | 2.97±2.29 | |||||||
| IIIA, IIIB, IVA | 8,787±3,285 | 6,111±2,922 | 67.4±10.4 | 1,866±606 | 23.1±8.8 | 3.53±1.88 | |||||||
| Tumor size (mm) | <0.001 | <0.001 | <0.001 | 0.074 | <0.001 | <0.001 | |||||||
| ≤50 | 7,437±2,341 | 4,783±2,065 | 62.8±10.9 | 1,993±721 | 28.0±9.4 | 2.77±2.15 | |||||||
| >50 | 8,875±3,103 | 6,158±2,729 | 67.6±10.5 | 1,865±664 | 22.7±8.9 | 3.67±2.12 | |||||||
| Histology | 0.237 | 0.213 | 0.321 | 0.281 | 0.089 | 0.13 | |||||||
| SCC | 8,089±2,790 | 5,406±2,475 | 65.0±11.0 | 1,930±690 | 25.5±9.5 | 3.20±2.23 | |||||||
| Others | 7,469±2,549 | 4,783±2,186 | 62.8±10.3 | 2,084±815 | 28.8±9.4 | 2.53±1.23 | |||||||
| LN metastasis | 0.143 | 0.018 | 0.004 | 0.012 | <0.001 | 0.047 | |||||||
| No | 7,751±2,496 | 5,008±2,209 | 62.9±11.4 | 2,051±747 | 28.0±9.9 | 2.88±2.45 | |||||||
| Yes | 8,248±2,939 | 5,605±2,592 | 66.2±10.4 | 1,866±656 | 24.3±9.0 | 3.33±1.96 | |||||||
| Treatment result | 0.002 | <0.001 | 0.001 | 0.026 | <0.001 | 0.001 | |||||||
| CR | 7,889±2,698 | 5,205±2,379 | 64.2±11.0 | 1,961±714 | 26.4±9.5 | 3.03±2.16 | |||||||
| PD | 9,487±3,085 | 6,810±2,730 | 70.4±9.4 | 1,753±515 | 20.0±7.8 | 4.21±2.09 | |||||||
WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio; FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; LN, lymph node; CR, complete remission; PD, persistent disease.
3. Survival and prognostic analysis
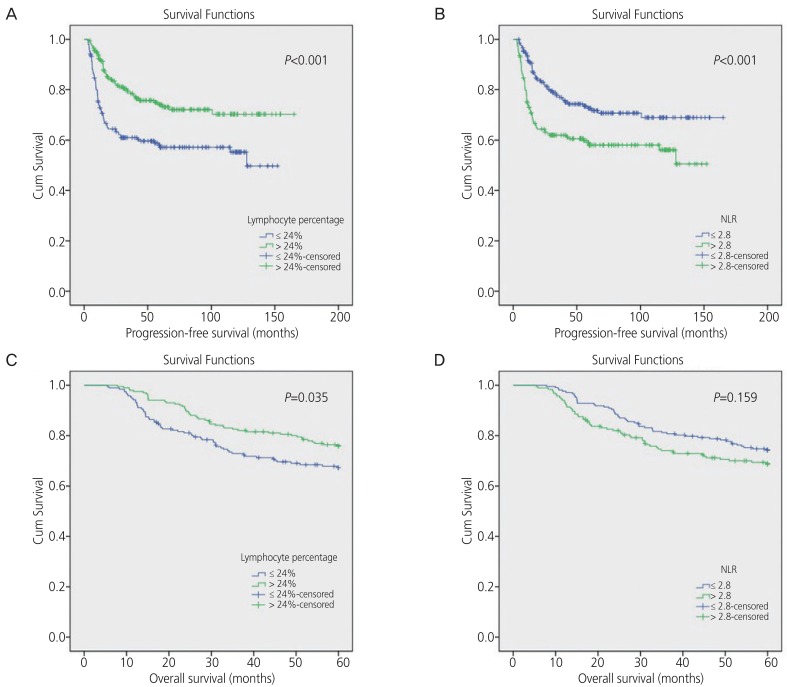
The median follow-up duration was 63.4 (range, 4–165) months. The 5-year PFS and OS rate for all patients was 65% and 72%, respectively. ROC curve analysis was performed for recurrence within two years for the patients who were not censored within that period (n=350). The cut-off values with the best discriminatory power were 2.8 for NLR and 24% for lymphocyte percentage, which were close to the median values of each variable (median NLR: 2.7, median lymphocyte percentage: 24%). Univariate analysis for PFS showed that patient age, stage, tumor size, histology, LN metastasis, ANC, neutrophil percentage, lymphocyte percentage, and NLR had prognostic significance (Table 4). Univariate analysis for OS showed that tumor size, LN metastasis, CCRT, and lymphocyte percentage had prognostic significance. Among the six hematologic markers, only lymphocyte percentage had statistical significance for both PFS and OS (Fig. 2).
Table 4. Univariate and multivariate analysis for progression-free survival and overall survival.
| Variables | Progression-free survival | Overall survival | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Age group | ||||||||||||||
| ≤50 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| 51–60 | 1.01 | 0.67–1.53 | 0.947 | 1.03 | 0.67–1.57 | 0.905 | 0.76 | 0.47–1.21 | 0.245 | 0.69 | 0.44–1.11 | 0.127 | ||
| >60 | 0.54 | 0.35–0.85 | 0.007 | 0.61 | 0.37–1.01 | 0.054 | 0.69 | 0.44–1.07 | 0.099 | 0.56 | 0.34–0.94 | 0.027 | ||
| FIGO stage | ||||||||||||||
| IIB | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| IIIA, IIIB, IVA | 1.46 | 1.02–2.08 | 0.039 | 1.23 | 0.84–1.80 | 0.295 | 1.35 | 0.91–1.99 | 0.135 | 1.24 | 0.82–1.89 | 0.307 | ||
| Tumor size (mm) | ||||||||||||||
| ≤50 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| >50 | 1.63 | 1.15–2.30 | 0.006 | 1.30 | 0.88–1.92 | 0.184 | 1.68 | 1.16–2.45 | 0.007 | 1.40 | 0.92–2.11 | 0.120 | ||
| Histology | ||||||||||||||
| SCC | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| Others | 2.18 | 1.27–3.74 | 0.005 | 2.67 | 1.51–4.58 | 0.001 | 1.84 | 0.99–3.44 | 0.055 | 2.13 | 1.13–4.02 | 0.020 | ||
| LN metastasis | ||||||||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| Yes | 1,62 | 1.12–2.35 | 0.011 | 1.33 | 0.88–2.00 | 0.181 | 1.82 | 1.20–2.75 | 0.004 | 1.66 | 1.06–2.61 | 0.027 | ||
| Treatment | ||||||||||||||
| CCRT | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| RT only | 1.09 | 0.73–1.63 | 0.675 | 1.42 | 0.91–2.21 | 0.121 | 1.95 | 1.32–2.86 | <0.001 | 2.60 | 1.68–4.03 | <0.001 | ||
| Lymphocyte percentage (%) | ||||||||||||||
| ≤24 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| >24 | 0.50 | 0.347–0.712 | <0.001 | 0.59 | 0.40–0.85 | 0.005 | 0.67 | 0.46–0.97 | 0.036 | 0.83 | 0.56–1.26 | 0.384 | ||
| Hematologic variables other than lymphocyte percentage | ||||||||||||||
| WBC count (n/µL) | ||||||||||||||
| ≤7,680 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| >7,680 | 1.26 | 0.89–1.79 | 0.189 | 1.13 | 0.79–1.62 | 0.500 | 1.14 | 0.78–1.65 | 0.505 | 0.98 | 0.66–1.44 | 0.900 | ||
| ANC (n/µL) | ||||||||||||||
| ≤5,110 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| >5,110 | 1.58 | 1.11–2.25 | 0.011 | 1.39 | 0.96–2.00 | 0.082 | 1.43 | 0.89–2.10 | 0.064 | 0.82 | 0.55–1.22 | 0.323 | ||
| Neutrophil percentage (%) | ||||||||||||||
| ≤65 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| >65 | 1.58 | 1.10–2.24 | 0.012 | 1.34 | 0.94–1.93 | 0.112 | 1.34 | 0.94–2.00 | 0.099 | 0.98 | 0.67–1.45 | 0.930 | ||
| ALC (n/µL) | ||||||||||||||
| ≤1,870 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| >1,870 | 0.78 | 0.55–1.10 | 0.159 | 0.90 | 0.63–1.29 | 0.557 | 0.88 | 0.60–1.28 | 0.506 | 0.98 | 0.67–1.45 | 0.930 | ||
| NLR | ||||||||||||||
| ≤2.8 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| >2.8 | 1.86 | 1.31–2.65 | 0.001 | 1.55 | 1.07–2.25 | 0.022 | 1.31 | 0.90–1.91 | 0.160 | 1.02 | 0.68–1.53 | 0.929 | ||
Multivariate anlysis was performed seperately for each hematologic variable adjusting all clinical variables. HR, hazard ratio; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; LN, lymph node; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; WBC, white blood cell; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NLR, neutrophil-to-lymphocyte ratio.
Fig. 2. Kaplan-Meier curve of progression-free survival stratified by lymphocyte percentage (A) and neutrophil-to-lymphocyte ratio level (B), overall survival stratified by lymphocyte percentage (C) and neutrophil-to-lymphocyte ratio level (D).
NLR, neutrophil-to-lymphocyte ratio.
As all hematologic variables were interrelated, 6 separate sets of multivariate analyses adjusting age, stage, tumor size, histology, LN metastasis, and treatment modality were established for each hematologic variable. Multivariate analysis adjusting all clinical variables revealed that lymphocyte percentage (hazard ratio [HR], 0.59; 95% confidence interval [CI], 0.40–0.85); P-value=0.005) and NLR (HR, 1.46; 95% CI, 1.01–2.43; P-value=0.047) were independent prognostic factors for PFS. None of the six hematologic variables remained significant for OS.
Multivariate analysis incorporating lymphocyte percentage showed that older patients had a better prognosis than that of younger patients. Similarly, older patients had a lower risk of death than younger patients, after adjusting for all clinical variables and lymphocyte percentage (HR, 0.56; 95% CI, 0.34–0.94).
Discussion
In this study, we found that patient age and various hematologic variables were associated with tumor extent, treatment outcomes, and risk of recurrence and death. Compared to old patients, younger patients had a larger tumor and higher incidence of LN metastasis. Although younger patients received CCRT more frequently than the older patients, treatment failure was more common in the younger patients. These patients had higher ANC, lower ALC, and higher NLR. A more advanced stage, larger tumor, LN metastasis, and treatment failure were associated with higher neutrophil and lower lymphocyte component. Among the hematologic variables, only lymphocyte percentage had a prognostic significance for both PFS and OS in univariable analysis. Compared to old patients, young patients had a higher risk of recurrence and death.
Aging and host immune function are known to be interrelated [23]. In the general healthy population, the population of lymphocytes and its subsets decreases with aging [24]. According to the immunosurveillance theory, a clinically viable tumor develops when the immune system fails to eradicate a single tumor cell [25]. This theory is supported by the increase in cancer risk with aging [26]. It has been reported that the number of lymphocytes and their subsets decrease with aging [24]. In cervical cancer, impaired host immunologic status is responsible for persistent human papillomavirus infection and resultant carcinogenesis [27]. Interestingly, ALC and age showed a positive correlation in our cohort, in contrast to the findings in the general population. Our findings that young patients with LACC had pretreatment leukocytosis, neutrophilia, relative lymphocytopenia, high NLR, more aggressive tumor, and poor response to RT match with the findings of previous studies [14,17,28,29]. These findings may imply that cervical cancer development at a relatively young age is associated with early escape from immunosurveillance resulting from host immune suppression. The escape from immunosurveillance may be responsible for carcinogenesis at a young age.
The occurrence of both neutrophilia and relative lymphocytopenia concurrent with malignancy are known to indicate host immune suppression. Suggested mechanisms of neutrophilia include a release of granulocyte colony-stimulating factor (G-CSF) by tumor cells, and cancer inflammation through release of interleukin-1 and tumor necrosis factor alpha [30,31]. It has been suggested that tumor-related leukocytosis and myeloid-derived suppressor cells induced by tumor-derived G-CSF are responsible for the rapidly progressive and radioresistant nature of tumors [31]. Lymphocytopenia represents a considerable decline in the cell-mediated immune system, demonstrated by marked decreases in T4 helper and T8 suppressor lymphocyte numbers [32].
The most well-established host immune parameter in patients with malignancy is NLR. A recent meta-analysis has reported that NLR had a greater magnitude of association with PFS and OS in patients with advanced disease and in those who received definitive RT [33]. In 2016, Cho et al. [14] reported a large-scale retrospective study with 2,456 patients with LACC who were treated with definitive RT. Both tumor-related leukocytosis (>9,000/µL) and high NLR (>2.5) were prognostic for both locoregional failure-free survival (LFFS) and OS, while high NLR (>2.5) did not influence LFFS. As lymphocyte percentage reflects leukocytosis more directly than NLR does, it can be used as a single hematologic parameter reflecting host immune status relating to RT response.
While neutrophilia is regarded as an inappropriate host immune response induced by a tumor, lymphocytopenia may represent a proper host response against the tumor [27]. Tumor-infiltrating lymphocytes (TIL) have been associated with an absence of LN metastasis and improved outcomes in early-stage cervical cancer treated by surgery [34]. There is a lack of evidence on the association between TIL and response to RT in LACC, probably because sufficient tissue for evaluating TIL cannot be obtained in most cases of LACC. Lymphocyte percentage, along with NLR, is an easy and inexpensive index to identify patients who may have a poor response to RT.
None of the hematologic variables including NLR influenced the OS in our study, in contrast to previous studies [14,33]. This discrepancy can be explained by the difference in patient cohorts between the current study and previous studies. In our cohort, a substantial number of patients with recurrent or persistent cervical cancer were salvaged by the single or combined application of surgical resection of local recurrence, metastasectomy, or metastasis-directed irradiation [35]. Patients at high-risk for recurrence, defined by lymphocyte percentage and/or NLR, may benefit from close monitoring after primary treatment and active application of aggressive salvage therapy in case of treatment failure or recurrence.
The main limitation of this study is its retrospective study design. We could not obtain pretreatment performance status and detailed data on chemotherapy from the medical record review. These variables have been reported as independent predictors of PFS and OS in patients with LACC treated with RT [36].
In conclusion, our study indicates that lymphocyte percentage, apart from NLR, has independent prognostic value for prediction of recurrence in patients with LACC who are treated with definitive RT. Younger patients with LACC who have low lymphocyte percentage at diagnosis should be closely monitored after primary treatment. As cancer immunotherapy for recurrent cervical cancer has recently been adopted [37], our finding that pretreatment lymphocyte status has independent prognostic value may be useful in identifying appropriate treatment candidates and for designing future clinical immunotherapy trials.
Acknowledgements
This study was supported by a grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS RTR: 50458-2013).
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Ethical approval: The study was approved by the Institutional Review Board of Korea Cancer Center (IRB No. K-1504-002-017) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent: The Institutional Review Board has approved a waiver of informed consent.
References
- 1.Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017;49:292–305. doi: 10.4143/crt.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shim SH, Kim H, Sohn IS, Hwang HS, Kwon HS, Lee SJ, et al. Nationwide cervical cancer screening in Korea: data from the National Health Insurance Service Cancer Screening Program and National Cancer Screening Program, 2009–2014. J Gynecol Oncol. 2017;28:e63. doi: 10.3802/jgo.2017.28.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiebe E, Denny L, Thomas G. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2012;119(Suppl 2):S100–S109. doi: 10.1016/S0020-7292(12)60023-X. [DOI] [PubMed] [Google Scholar]
- 4.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kietlińska Z. T and B lymphocyte counts and blast transformation in patients with Stage I cervical cancer. Gynecol Oncol. 1984;18:247–256. doi: 10.1016/0090-8258(84)90033-7. [DOI] [PubMed] [Google Scholar]
- 7.Shazly SA, Murad MH, Dowdy SC, Gostout BS, Famuyide AO. Robotic radical hysterectomy in early stage cervical cancer: a systematic review and meta-analysis. Gynecol Oncol. 2015;138:457–471. doi: 10.1016/j.ygyno.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Choi CH, Kang H, Kim WY, Kim TJ, Lee JW, Huh SJ, et al. Prognostic value of baseline lymphocyte count in cervical carcinoma treated with concurrent chemoradiation. Int J Radiat Oncol Biol Phys. 2008;71:199–204. doi: 10.1016/j.ijrobp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Wu ES, Oduyebo T, Cobb LP, Cholakian D, Kong X, Fader AN, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol. 2016;140:76–82. doi: 10.1016/j.ygyno.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haraga J, Nakamura K, Omichi C, Nishida T, Haruma T, Kusumoto T, et al. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Mol Clin Oncol. 2016;5:567–574. doi: 10.3892/mco.2016.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Zhang F, Sheng XG, Zhang SQ, Chen YT, Liu BW. Peripheral platelet/lymphocyte ratio predicts lymph node metastasis and acts as a superior prognostic factor for cervical cancer when combined with neutrophil: lymphocyte. Medicine (Baltimore) 2016;95:e4381. doi: 10.1097/MD.0000000000004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onal C, Guler OC, Yildirim BA. Prognostic use of pretreatment hematologic parameters in patients receiving definitive chemoradiotherapy for cervical cancer. Int J Gynecol Cancer. 2016;26:1169–1175. doi: 10.1097/IGC.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Nishida T, Haruma T, Haraga J, Omichi C, Ogawa C, et al. Pretreatment platelet-lymphocyte ratio is an independent predictor of cervical cancer recurrence following concurrent chemoradiation therapy. Mol Clin Oncol. 2015;3:1001–1006. doi: 10.3892/mco.2015.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho Y, Kim KH, Yoon HI, Kim GE, Kim YB. Tumor-related leukocytosis is associated with poor radiation response and clinical outcome in uterine cervical cancer patients. Ann Oncol. 2016;27:2067–2074. doi: 10.1093/annonc/mdw308. [DOI] [PubMed] [Google Scholar]
- 15.Wang YY, Bai ZL, He JL, Yang Y, Zhao R, Hai P, et al. Prognostic value of neutrophil-related factors in locally advanced cervical squamous cell carcinoma patients treated with cisplatin-based concurrent chemoradiotherapy. Dis Markers. 2016;2016:3740794. doi: 10.1155/2016/3740794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizunuma M, Yokoyama Y, Futagami M, Aoki M, Takai Y, Mizunuma H. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Int J Clin Oncol. 2015;20:989–996. doi: 10.1007/s10147-015-0807-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW, Lee JH, et al. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res. 2012;32:1555–1561. [PubMed] [Google Scholar]
- 18.Zhang Y, Wang L, Liu Y, Wang S, Shang P, Gao Y, et al. Preoperative neutrophil-lymphocyte ratio before platelet-lymphocyte ratio predicts clinical outcome in patients with cervical cancer treated with initial radical surgery. Int J Gynecol Cancer. 2014;24:1319–1325. doi: 10.1097/IGC.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Wen TF, Yan LN, Li B, Yang JY, Xu MQ, et al. Scoring selection criteria including total tumour volume and pretransplant percentage of lymphocytes to predict recurrence of hepatocellular carcinoma after liver transplantation. PLoS One. 2013;8:e72235. doi: 10.1371/journal.pone.0072235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He JR, Shen GP, Ren ZF, Qin H, Cui C, Zhang Y, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck. 2012;34:1769–1776. doi: 10.1002/hed.22008. [DOI] [PubMed] [Google Scholar]
- 21.Yoneyama Y, Ito M, Sugitou M, Kobayashi A, Nishizawa Y, Saito N. Postoperative lymphocyte percentage influences the long-term disease-free survival following a resection for colorectal carcinoma. Jpn J Clin Oncol. 2011;41:343–347. doi: 10.1093/jjco/hyq223. [DOI] [PubMed] [Google Scholar]
- 22.Lee WM, Park SI, Kim BJ, Kim MH, Choi SC, Lee ED, et al. Clinicopathologic factors for central recurrence in patients with locally advanced bulky cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2012;161:219–223. doi: 10.1016/j.ejogrb.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Lin Y, Kim J, Metter EJ, Nguyen H, Truong T, Lustig A, et al. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immun Ageing. 2016;13:24. doi: 10.1186/s12979-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sansoni P, Cossarizza A, Brianti V, Fagnoni F, Snelli G, Monti D, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–2773. [PubMed] [Google Scholar]
- 25.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 26.Mendivil AA, Rettenmaier MA, Abaid LN, Brown JV, 3rd, Micha JP, Lopez KL, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol. 2016;25:66–71. doi: 10.1016/j.suronc.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Conesa-Zamora P. Immune responses against virus and tumor in cervical carcinogenesis: treatment strategies for avoiding the HPV-induced immune escape. Gynecol Oncol. 2013;131:480–488. doi: 10.1016/j.ygyno.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Monk BJ, Tian C, Rose PG, Lanciano R. Which clinical/pathologic factors matter in the era of chemoradiation as treatment for locally advanced cervical carcinoma? Analysis of two Gynecologic Oncology Group (GOG) trials. Gynecol Oncol. 2007;105:427–433. doi: 10.1016/j.ygyno.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau HY, Juang CM, Chen YJ, Twu NF, Yen MS, Chao KC. Aggressive characteristics of cervical cancer in young women in Taiwan. Int J Gynaecol Obstet. 2009;107:220–223. doi: 10.1016/j.ijgo.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Mabuchi S, Matsumoto Y, Isohashi F, Yoshioka Y, Ohashi H, Morii E, et al. Pretreatment leukocytosis is an indicator of poor prognosis in patients with cervical cancer. Gynecol Oncol. 2011;122:25–32. doi: 10.1016/j.ygyno.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 31.Mabuchi S, Matsumoto Y, Kawano M, Minami K, Seo Y, Sasano T, et al. Uterine cervical cancer displaying tumor-related leukocytosis: a distinct clinical entity with radioresistant feature. J Natl Cancer Inst. 2014;106:dju147. doi: 10.1093/jnci/dju147. [DOI] [PubMed] [Google Scholar]
- 32.Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. 2013;108:2116–2122. doi: 10.1038/bjc.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ethier JL, Desautels DN, Templeton AJ, Oza A, Amir E, Lheureux S. Is the neutrophil-to-lymphocyte ratio prognostic of survival outcomes in gynecologic cancers? A systematic review and meta-analysis. Gynecol Oncol. 2017;145:584–594. doi: 10.1016/j.ygyno.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 35.Kim TH, Kim MH, Kim BJ, Park SI, Ryu SY, Cho CK. Prognostic importance of the site of recurrence in patients with metastatic recurrent cervical cancer. Int J Radiat Oncol Biol Phys. 2017;98:1124–1131. doi: 10.1016/j.ijrobp.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 36.Nugent EK, Case AS, Hoff JT, Zighelboim I, DeWitt LL, Trinkhaus K, et al. Chemoradiation in locally advanced cervical carcinoma: an analysis of cisplatin dosing and other clinical prognostic factors. Gynecol Oncol. 2010;116:438–441. doi: 10.1016/j.ygyno.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 37.Minion LE, Tewari KS. Cervical cancer - state of the science: from angiogenesis blockade to checkpoint inhibition. Gynecol Oncol. 2018;148:609–621. doi: 10.1016/j.ygyno.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]