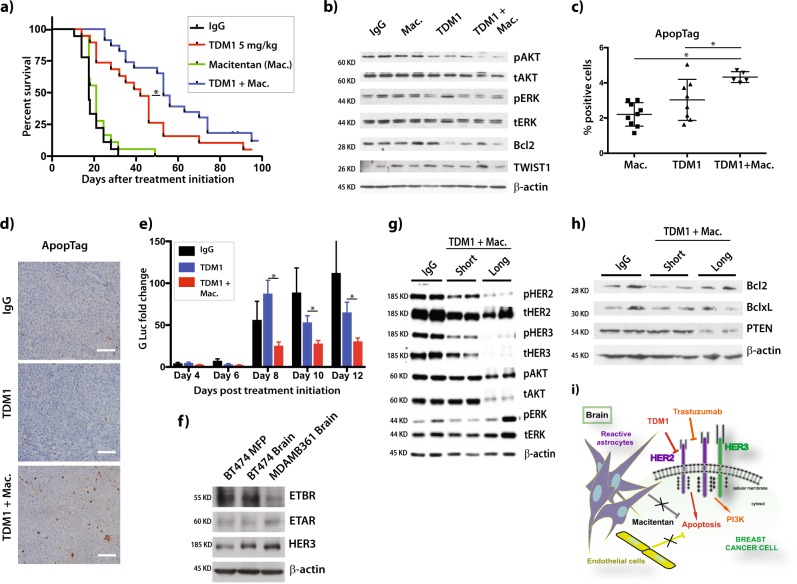
Fig. 1.
Effects of macitentan on the efficacy of T-DM1 against HER2-positive breast cancer tumors in the brain microenvironment. a Mouse survival in nude mice with BT474-Gluc brain tumors treated with i) control vehicle, T-DM1 (5 mg/kg, i.v. weekly), macitentan (50 mg/kg p.o. daily) or their combination (N = 18-21). b Western blots were performed in BT474-Gluc tumors, collected 48 h after treatment (blot samples were derived from the same experiment and were processed in parallel). c Cell apoptosis was determined in BT474-Gluc brain metastases after quantification of ApopTag, 5 days after treatment with control vehicle, T-DM1 (1 × 5 mg/kg), or T-DM1 (1 × 5 mg/kg) + macitentan (5 × 50 mg/kg) (N = 5-9). Error bars are standard deviation. d Representative images of BT474-Gluc tumors in the brain after staining for ApopTag. Scale bar = 0.1 mm. e Relative activity of Gaussia luciferase in the media of organotypic brain slice cultures of BT474-Gluc cells following treatment with T-DM1 alone or in combination with macitentan. Gluc levels from treated cells were normalized to Day 0 for each treatment. Error bars are standard deviation (N = 5–7). f Western blots were performed in BT474-Gluc tumors growing in the brain or the mammary fat pad (MFP), and in MDA-MD-361-Gluc brain tumors. HER3 was used as control, since it has been previously established that the brain microenvironment increases HER3 expression6 g, h. Western blots were performed in BT474-Gluc brain tumors, collected 48 h after treatment with T-DM1 + macitentan (T-DM1 + mac. short) and at the endpoint of the study (T-DM1 + mac. long). Tumors from mice treated with unspecific IgG were collected and used as control. i Schematic of brain-stroma mediated protection to the HER2-targeted ADC T-DM1. Direct contact of reactive astrocytes and brain endothelial cells with HER2-amplified breast cancer cells can reduce the activity of ado-trastuzumab emtansine (T-DM1) through an endothelin-pathway-mediated mechanism. Dual endothelin receptor inhibition with macitentan increases T-DM1 induced apoptosis and enhances the activity of the ADC in the brain microenvironment