Abstract
Aripiprazole is an antipsychotic drug characterized by partial agonist activity at D2 receptors to normalize both hyperdopaminergic and hypodopaminergic states. Traditional D2 antagonist antipsychotic drugs have been shown previously to reduce dopamine neuron activity through action on D2 autoreceptors to produce an overexcitation-induced cessation of cell firing, referred to as depolarization block. It is unclear whether aripiprazole reduces dopamine neuron activity via inhibition or, as seen following D2 antagonist administration, depolarization block. The impact of acute and repeated aripiprazole treatment was examined in the methylazoxymethanol acetate (MAM) rodent model to observe its effects on a hyperdopaminergic system, compared to normal rats. We found that administration of aripiprazole acutely or after 1 or 7 days of withdrawal from 21-day repeated treatment led to a decrease in the number of spontaneously active dopamine neurons in MAM rats but not in controls. This reduction was not reversed by apomorphine (100–200 µg/kg i.p. or 20 µg/kg i.v.) administration, suggesting that it was not due to depolarization block. In contrast, 1 h after induction of depolarization block of dopamine neurons by acute haloperidol treatment (0.6 mg/kg i.p.), aripiprazole (1 mg/kg, i.p.) reversed the depolarization block state. Therefore, aripiprazole rapidly reduced the hyperdopaminergic activity selectively in MAM rats. The reduction is unlikely due to depolarization block and persists following 7-day withdrawal from repeated treatment. Aripiprazole also removes haloperidol-induced depolarization block in MAM rats, which may underlie the acute psychotic state often observed with switching to this treatment.
Subject terms: Pharmacology, Schizophrenia, Neurophysiology
The majority of current antipsychotic drugs (APDs) are D2 receptor antagonists [1, 2]. A mechanism proposed for the therapeutic action of D2 receptor antagonists on dopamine (DA) dysregulation observed in schizophrenia [3] is DA neuron depolarization block [4, 5]. Following repeated treatment with D2 receptor antagonists, a substantially depolarized membrane potential results in overexcitation-induced cessation of spiking [4] primarily due to blockade of somatodendritic D2 autoreceptors that provide local feedback inhibition of spike activity [6, 7]. Depolarization block can be reversed by administration of a DA agonist, such as apomorphine. At low doses, apomorphine acts preferentially at D2 autoreceptors to inhibit DA neurons in depolarization block sufficiently to restore spiking activity, consistent with an APD-induced overexcitation rather than inhibition of DA neuron firing [4, 8]. The net result of depolarization block is a reduced number of spontaneously active DA neurons [4, 8–10] available for phasic activation [11] and striatal DA release [12].
Following prolonged D2 antagonist treatment, a compensatory upregulation of D2 receptors has been observed in humans and animal models that persists after APD discontinuation [13–17]. The increase in D2 receptors is associated with increased behavioral sensitivity to DA. It has been proposed that DA supersensitivity underlies the relapse of psychotic symptoms following discontinuation of treatment or dose reduction, which may include the appearance of new or more severe psychotic symptoms [18, 19]. In animal models, increased striatal D2 receptor density is associated with an increased locomotor response to amphetamine and DA agonists following withdrawal from repeated APD administration [20–23]. Although numerous studies have associated the emergence of DA supersensitivity to increased D2 receptor density, the nature of the relationship is not clearly understood. There have been examples of increased D2 receptors during ongoing APD treatment without behavioral evidence of DA supersensitivity [22, 23] and chronic APD treatment regimens that have not resulted in D2 receptor upregulation [24], suggesting a complex relationship based potentially on factors including dose and treatment regimen.
In contrast to the D2 receptor antagonism observed with other APDs, aripiprazole (ARI) is a D2 receptor partial agonist. The unique mechanism of action of ARI demonstrates properties of a functional agonist or antagonist in animal models of DA hypoactivity and hyperactivity, respectively, thus providing stabilization of the DA system [25, 26]. In contrast to both first-generation APDs and other second-generation APDs, there is evidence that ARI may not induce upregulation of D2 receptors or DA supersensitivity [17, 27]. Due to its unique characteristics, including absence of DA supersensitivity following withdrawal from chronic treatment [17], we hypothesized that ARI does not induce DA neuron depolarization block.
Animal models provide a useful tool to study the state-dependent action of drugs that may not be readily observed in a normal system. To model the hyperdopamineregic state characteristic of schizophrenia we have utilized methylazoxymethanol acetate (MAM) rats, born with a developmental disruption induced through administration of this DNA alkylating agent to pregnant dams at gestational day 17 (GD17). Adult offspring of MAM-treated rats demonstrate anatomical, behavioral, pharmacological, and physiological abnormalities consistent with phenotypes observed in patients with schizophrenia [28–31] As a consequence of hippocampal hyperactivity, also observed in patients with schizophrenia [32–34], MAM rats display increased DA neuron population activity [28] that rapidly results in depolarization block following acute administration of first- and second-generation APDs [35]. MAM rats thus provide a clinically relevant model to study state-dependent effects of acute and repeated ARI administration on the DA system with the aim of clarifying the range of action of ARI at D2 receptors.
Methods
Subjects
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals by the United States Public Health Service and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Timed pregnant Sprague-Dawley dams (Envigo, Indianapolis, IN) were obtained on GD15 and MAM (20 mg/kg, intraperitoneal (i.p.), Midwest Research Institute, Kansas City, MO) or saline (SAL; 1 ml/kg, i.p.) was administered on GD17. The male pups were weaned on postnatal day 23 and pair-housed with littermates in a temperature (22 °C)- and humidity (47%)-controlled facility on a 12 h light/dark cycle (lights on 7 AM to 7 PM) with ad libitum food and water. Rats were used for experiments at 3–6 months of age (≥300 g).
Antipsychotic drug administration
Acute and repeated aripiprazole administration
MAM and SAL rats were assigned randomly to treatment groups of ARI (3 mg/kg or 10 mg/kg; Sigma-Aldrich, St. Louis, MO) or vehicle (VEH). Treatments were administered orally using palatable vanilla wafers to which the rats were habituated for 2 days prior to treatment. Oral administration was used to circumvent injection stress and better approximate the clinical protocol [36], as previously described [20, 37]. ARI was placed on a wafer containing 0.1 ml liquid sugar and dissolved into the wafer with an additional 0.1–0.2 ml liquid sugar. VEH-treated rats were administered the same preparation without drug. Rats were placed individually into transport tubs to consume the wafers (usually <10 min) before being returned to home cages.
For acute treatments, ARI or VEH wafers were consumed 2 h prior to the start of electrophysiological recording to observe effects at peak brain concentration as reported previously following oral administration in rats [38]. For repeated treatments, ARI and VEH wafers were administered at approximately 9 AM daily for 21 days and rats were recorded either the following day or after 7-day withdrawal.
Acute haloperidol administration
MAM rats were injected with haloperidol (HAL; 0.6 mg/kg, i.p.; Sigma-Aldrich) dissolved in 0.23% glacial acetic acid in distilled H2O and returned to their home cage. Recordings took place 1 h following HAL administration, which was shown to be sufficient time to induce depolarization block in MAM rats [35].
Electrophysiological recordings
The activity state of DA neurons in the ventral tegmental area (VTA) was measured using in vivo extracellular recordings. Rats were anesthetized with chloral hydrate (400 mg/kg; i.p.) and placed on a stereotaxic frame (Kopf, Tujunga, CA). Supplemental anesthesia was administered i.p. to maintain suppression of the hind limb withdrawal reflex. Body temperature was maintained at 37 °C with a temperature-controlled heating pad (CWE Inc., Ardmore, PA). Single-barrel glass electrodes (WPI, Sarasota, FL) were pulled vertically (PE-2, Narasige, Japan), broken under a microscope to an impedance of 6–8 MΩ, and filled with 2 M NaCl containing 2% Chicago Sky Blue dye in 2 M saline. Electrodes were lowered through the VTA from 5.3–5.7 mm posterior, 0.6–1.0 mm lateral, and 6.5–9.0 mm ventral from the top of brain via a hydraulic micropositioner (Kopf). Single-unit activity was obtained using an amplifier (Fintronics, Orange, CT) using a highpass filter at 30 Hz and lowpass at 16 kHz. DA neurons were classified based on established criteria, including a biphasic action potential with duration >2.2 ms, a slow firing rate (1–10 Hz), and irregular and burst firing patterns with burst onset defined as an interspike interval <80 msec and burst termination by a subsequent interspike interval >160 msec [39–41]. Recordings were performed by making 6–9 vertical electrode passes (“tracks”) in a predetermined grid pattern with each track separated by 0.2 mm. The activity of each DA neuron was recorded for at least 1 min of stable spontaneous activity with a signal-to-noise ratio greater than 2:1 using LabChart software (AD Instruments, Colorado Springs, CO). Three parameters were analyzed: (1) the average number of spontaneously active DA neurons encountered per electrode track (“population activity”), (2) average firing rate, and (3) the percentage of spikes that occurred in bursts (%SIB).
In a subset of rats, after the first six tracks rats were administered apomporphine (100–200 µg/kg i.p. or 20 µg/kg intravenous (i.v.), in saline; Sigma-Aldrich) for acute and repeated aripiprazole recordings or aripiprazole (1 mg/kg in saline i.p.; Sigma-Aldrich) for acute HAL recordings. Apomorphine was administered in incrementally increasing doses until approximately a 50% decrease in baseline firing rate or bursting activity was detected in the DA neuron. Immediately following i.v. administration or 10 min following i.p. administration, an additional six tracks were recorded.
Histology
Electrode placement was verified following each experiment via electrophoretic ejection of Chicago Sky Blue dye from the tip of the recording electrode (−20 µA constant current, 20 min). Rats were then overdosed with chloral hydrate and decapitated. The brains were removed and fixed for at least 48 h (8% paraformaldehyde in phosphate-buffered saline (PBS)), cryoprotected (25% sucrose in PBS) until saturated, and sliced on a cryostat into 60 µm sections which were mounted onto gelatin-coated slides. Slides were stained with a mixture of cresyl violet and neutral red for verification of electrode sites with reference to a stereotaxic atlas [42].
Analysis
Analysis of firing rate and bursting activity was performed using NeuroExplorer (Plexon, Dallas, TX). Population activity (“cells/track”) was averaged within each animal and then across animals in each group, whereas the firing rate and burst activity of each neuron was counted as an independent replicate and averaged across animals in a group. Significance for acute and repeated aripiprazole experiments was assessed with a two-way analysis of variance (ANOVA) (MAM × Treatment) followed by Tukey's post hoc comparisons. Significance for depolarization block experiments was assessed with a two-tailed, paired t-test and the Wilcoxon signed-rank test was used for non-normally distributed data. Significance for the haloperidol experiment was assessed with a one-way ANOVA followed by Tukey's post hoc and cells/track comparisons were made with a two-tailed paired t-test. All statistics were calculated using SigmaPlot (Systat Software, San Jose, CA) and all data are represented as the mean ± SEM.
Results
Acute aripiprazole administration reduces VTA DA neuron activity of MAM but not SAL rats
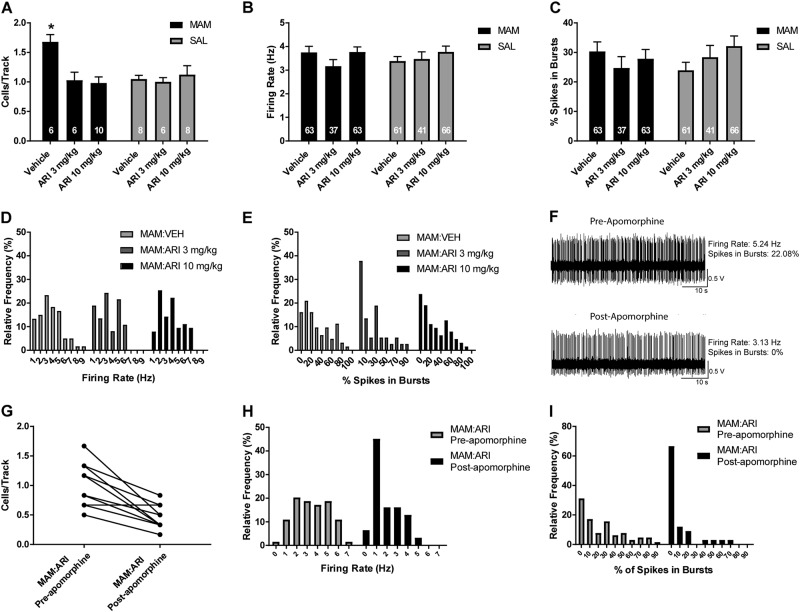
Electrophysiological recordings were conducted from MAM rats and SAL rats, with each group receiving either VEH or ARI (3 mg/kg or 10 mg/kg, p.o.). VEH-treated MAM rats (n = 6 rats, 63 neurons) exhibited the anticipated elevation in population activity with an average of 1.7 ± 0.1 cells/track compared to VEH-treated SAL rats (n = 8 rats, 61 neurons) which had an average of 1.1 ± 0.1 cells/track (Fig. 1a; 2-way ANOVA main effects: for MAM F(2, 40) = 3.189, p = 0.010; for ARI F(2, 40) = 5.049, p = 0.011; MAM-by-ARI interaction F(2, 40) = 5.882, p = 0.005; post hoc MAM control vs SAL control p = 0.001). Acute ARI treatment significantly reduced DA neuron population activity in MAM rats, both at 3 mg/kg (n = 6 rats, 37 neurons; post hoc MAM control vs MAM 3 mg/kg: p = 0.007) and 10 mg/kg (n = 10 rats, 63 neurons; post hoc MAM control vs MAM 10 mg/kg: p = 0.001) compared to VEH-treated MAM rats. In contrast, there was no reduction in DA neuron population activity in ARI-treated SAL rats, at 3 mg/kg (n = 6, 41 neurons) or 10 mg/kg (n = 8 rats, 66 neurons), compared to VEH-treated SAL rats (Fig. 1a). There was no significant change in firing rate (Fig. 1b) or bursting (Fig. 1c) with ARI treatment compared to VEH treatment in MAM or SAL rats. There was also no overt shift in the distribution of firing rate or bursting (Fig. 1d, e), despite the reduction in population activity observed in MAM rats. In ARI-treated MAM rats, at both 3 mg/kg and 10 mg/kg, a systemic injection of apomorphine (100–200 µg/kg i.p.) reduced firing rate and bursting activity of DA neurons held during injection. In an example neuron, apomorphine reduced firing rate of a DA neuron from 5.2 Hz to 3.1 Hz and the percentage of spikes in burst from 22.2 to 0% 5 min following the injection (Fig. 1f). An additional six tracks were recorded following apomorphine injection, without significant change in population activity compared to tracks recorded before apomorphine (Fig. 1g) and a leftward shift in the distribution of the firing rate and bursting activity of recorded DA neurons recorded after apomorphine compared to before apomorphine (Fig. 1h–i).
Fig. 1.
Acute administration of ARI reduced DA neuron activity in the VTA in MAM but not SAL rats in a manner distinct from D2 receptor antagonist antipsychotic drugs [35]. a At 2 h following oral ARI administration of 3 mg/kg and 10 mg/kg, MAM rats displayed a reduced number of spontaneously active DA neurons in the VTA compared to MAM rats that received VEH administration, which was not observed in SAL rats. There were no significant differences in the firing rate (b, c) or percentage of spikes in bursts (d, e) between ARI and VEH administration in MAM or SAL rats. f An example trace of a DA neuron recorded before and 5 min after APO, which was administered i.p. following six tracks, 10 min prior to recording an additional six tracks. APO reduced the firing rate and bursting activity of the DA neuron. g APO administration did not significantly reduce DA neuron population activity in the VTA of ARI-treated MAM rats, although there was a trend toward reduced spontaneous activity and a leftward shift in the distribution of firing rate (h) and percent of spikes in bursts (i) in neurons recorded after APO compared to tracks recorded before APO. *p < 0.05
Repeated aripiprazole administration reduces VTA DA neuron activity of MAM but not SAL rats
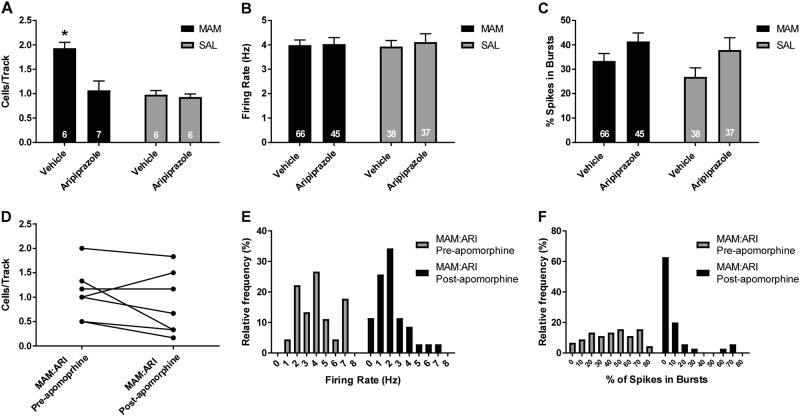
To evaluate the effects of repeated ARI treatment, MAM and SAL rats received 21 days of ARI (10 mg/kg) or VEH daily treatment p.o. and their VTA activity was recorded the following day. VEH-treated MAM rats (n = 6 rats, 66 neurons) displayed a significant elevation in DA neuron population activity (average 1.9 ± 0.1 cells/track) compared to VEH-treated SAL rats (Fig. 2a; n = 6 rats, 38 neurons; average 1.0 ± 0.1 cells/track; 2-way ANOVA main effects: for MAM F(1, 21) = 16.401, p < 0.001; for ARI F(1, 21) = 11.602, p = 0.003; MAM-by-ARI interaction F(1, 21) = 9.223, p = 0.006; post hoc MAM control vs SAL control p < 0.001). Repeated ARI treatment significantly reduced DA neuron population activity in ARI-treated MAM rats (n = 7 rats, 45 cells) compared to VEH-treated MAM rats to an average of 1.1 ± 0.1 cells/track (Fig 2a; 2-way ANOVA; post hoc MAM ARI vs MAM control: p < 0.001). In contrast, there was no reduction in DA neuron population activity in ARI-treated SAL rats (Fig. 2a; n = 6 rats, 37 neurons; average 0.9 ± 0.1 cells per track), compared to VEH-treated SAL rats (Fig. 2a). There was no significant change in firing rate with repeated ARI treatment compared to VEH treatment in MAM or SAL rats (Fig. 2b; control baseline: 3.9 ± 0.3 Hz; control ARI: 4.1 ± 0.3 Hz; MAM baseline: 4.0 ± 0.2 Hz; MAM ARI: 4.0 ± 0.3 Hz), and a main effect of ARI treatment on bursting activity in MAM and SAL rats (Fig. 2c; control baseline: 26.9 ± 4.2%; control ARI: 37.9 ± 4.3%; MAM baseline: 33.4 ± 3.0%; MAM ARI: 41.4 ± 3.5%; 2-way ANOVA F(1, 193) = 6.016, p = 0.015). In MAM rats that received repeated ARI treatment, a systemic injection of apomorphine (200 µg/kg i.p.) resulted in no significant change in DA neuron population activity in an additional six tracks that were recorded 10 min following the injection compared to tracks recorded before apomorphine (Fig. 2d); a leftward shift in the firing rate and bursting activity of DA neurons recorded after apomorphine compared to before apomorphine was also observed (Fig. 2e, f).
Fig. 2.
Repeated administration of ARI for 21 days p.o. reduced DA neuron activity in the VTA in MAM but not SAL rats in a manner distinct from D2 receptor antagonist antipsychotic drugs [4, 10, 35]. a At 24 h following 21-day repeated ARI treatment, MAM rats displayed a reduced number of spontaneously active DA neurons in the VTA compared to MAM rats that received VEH administration, which was not observed in SAL rats. There was no significant difference in the firing rate (b) of DA neurons between ARI and VEH administration in MAM or SAL rats and there was a main effect of ARI treatment on the percentage of spikes in bursts (c). In MAM rats that received ARI, APO was administered i.p. following six tracks, 10 min prior to recording an additional six tracks. d APO administration did not significantly reduce DA neuron population activity in the VTA of ARI-treated MAM rats and produced a leftward shift in the distribution of firing rate (e) and percent of spikes in burst (f) in DA neurons recorded after APO compared to tracks recorded before APO. *p < 0.05
Persistent reduction of VTA DA neuron activity following withdrawal from repeated aripiprazole administration
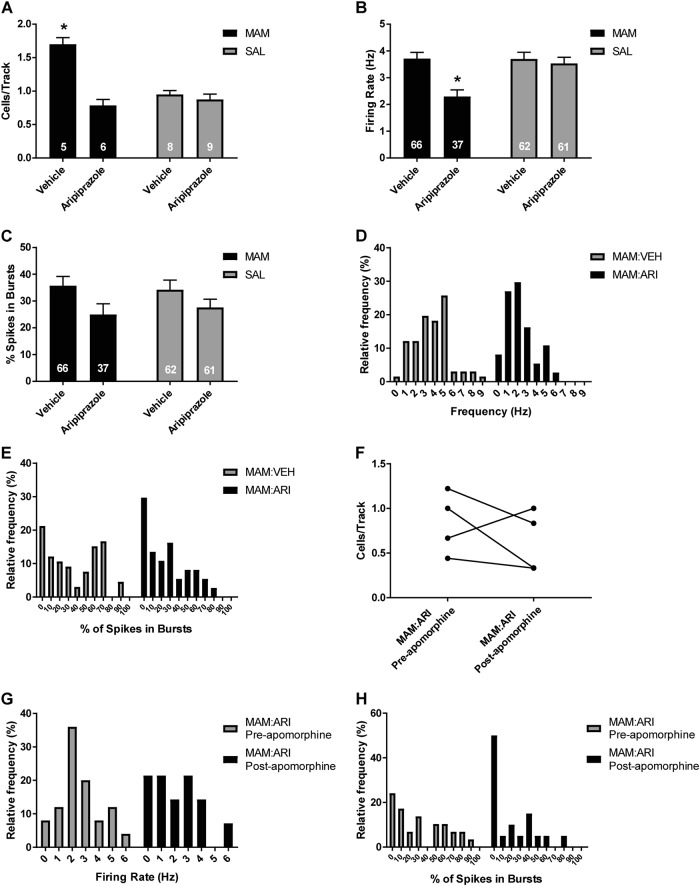
Given our findings that acute and repeated ARI treatment results in a reduction in VTA DA neuron activity in MAM rats that is not restored by apomorphine administration, we examined whether this reduction remained following an extended 7-day withdrawal from 21-day repeated treatment. VEH-treated MAM rats (n = 5 rats, 66 neurons) displayed a significant elevation in DA neuron population activity with an average of 1.7 ± 0.1 cells/track, compared to VEH-treated SAL rats (n = 8 rats, 62 neurons), with an average of 1.0 ± 0.1 cells/track (Fig. 3a; 2-way ANOVA main effects: for MAM F(1, 24) = 16.024, p < 0.001; for ARI F(1, 24) = 35.985, p < 0.001; MAM-by-ARI interaction F(1, 24) = 25.671, p < 0.001; post hoc MAM control vs SAL control p < 0.001). DA neuron population activity remained reduced in MAM rats following 7-day withdrawal from 21-day repeated ARI treatment (n = 6 rats, 37 neurons) compared to VEH-treated MAM rats with an average of 0.8 ± 0.1 cells/track and of spikes in bursts (Fig 3a; 2-way ANOVA; post hoc MAM ARI vs MAM control: p < 0.001). In contrast, there was no reduction in DA neuron population activity in ARI-treated SAL rats (n = 9 rats, 61 neurons), with an average of 0.9 ± 0.1 cells/track (Fig. 3a). MAM rats withdrawn from repeated ARI treatment displayed a significant reduction in firing rate (Fig. 3b; control baseline: 3.7 ± 0.2 Hz; control ARI: 3.5 ± 0.2 Hz; MAM baseline: 3.7 ± 0.2 Hz; MAM ARI: 2.3 ± 0.3 Hz; 2-way ANOVA; main effects: for MAM F(1, 222) = 6.029, p = 0.015, for ARI F(1, 222) = 10.019, p = 0.002; MAM-by-ARI interaction F(1, 222) = 6.268, p = 0.013; post hoc MAM ARI vs MAM control p < 0.001) and there was a main effect of ARI treatment on bursting activity of VTA DA neurons in MAM and SAL rats withdrawn from repeated ARI treatment (Fig. 3c; control baseline: 34.2 ± 3.4; control ARI; 27.6 ± 3.4% MAM baseline: 35.7 ± 3.3%; MAM ARI: 25.0 ± 4.3%; 2-way ANOVA; main effect for ARI F(1, 222) = 5.772, p = 0.017), which was reflected in the distribution of DA neurons recording in ARI-treated MAM rats compared to VEH-treated MAM rats (Fig. 3d, e). In MAM rats withdrawn from repeated ARI treatment, a systemic injection of apomorphine (200 µg/kg i.p. or 20 µg/kg, i.v.) did not cause a significant change in DA neuron population activity in an additional six tracks that were recorded 10 min following the i.p. injection (n = 3 rats) or immediately following an i.v. injection (n = 1 rat) compared to tracks recorded before apomorphine (Fig. 3f). There was also a leftward shift in the firing rate and, more prominently, bursting activity of DA neurons recorded after apomorphine compared to before apomorphine (Fig. 3g, h).
Fig. 3.
Spontaneous DA neuron activity in the VTA of MAM rats remains reduced following 7-day withdrawal from 21-day ARI treatment in a manner distinct from depolarization block. a MAM rats displayed reduced VTA DA neuron following 7-day withdrawal from 21-day repeated ARI treatment compared to VEH treatment, which was not observed in SAL rats. b MAM rats withdrawn from repeated ARI treatment displayed a reduced firing rate of DA neurons compared to VEH-treated MAM rats, which was not observed in SAL rats. c ARI treatment reduced the percentage of spikes in bursts in both MAM and SAL rats compared to VEH-treated rats. This was evident as a leftward shift in the distribution of both firing rate (d) and bursting activity (e) of DA neurons in ARI-treated MAM rats compared to VEH-treated MAM rats. In MAM rats that received ARI, APO was administered i.p. or i.v. following six tracks, prior to recording an additional six tracks. f APO administration did not significantly change DA neuron population activity in the VTA of ARI-treated MAM rats and produced a leftward shift in the distribution of firing rate (g) and percent of spikes in burst (h) in DA neurons recorded after APO compared to tracks recorded before APO. *p < 0.05
Acute aripiprazole administration reverses haloperidol-induced depolarization block in MAM rats
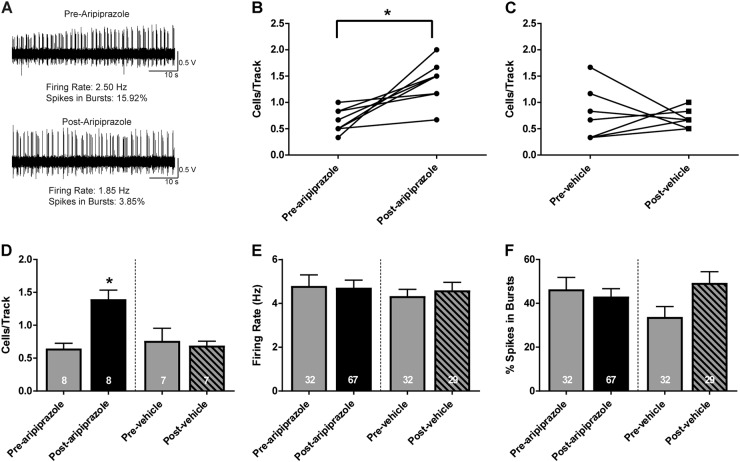
To determine the effect of acute ARI administration on depolarization block, six tracks were recorded in the VTA of MAM rats 1 h following acute treatment with haloperidol (0.6 mg/kg, i.p.) and an additional six tracks were recorded 10 min following acute ARI administration (1 mg/kg, i.p.). An example DA neuron recorded before ARI administration displayed an average firing rate of 2.5 Hz with 15.9% of spikes fired in bursts. The same neuron displayed a firing rate of 1.9 Hz with 3.9% of spikes fired in bursts following ARI administration (Fig. 4a). There was a significant increase in the number of spontaneously active DA neurons recorded after acute ARI administration (1.4 ± 0.1 cells/track) compared to before (0.6 ± 0.1 cells/track; paired t-test; t(7) = −4.031, p = 0.005). In contrast, there was no change in DA neuron population activity in tracks recorded after VEH administration (1 ml/kg saline, i.p., 0.7 ± 0.2 cells/track) compared to before (0.8 ± 0.2 cells/track; Fig. 4b–d). There was no significant difference in the firing rate (Fig. 4e) or bursting activity (Fig. 4f) between groups.
Fig. 4.
Reversal of haloperidol-induced depolarization block in MAM rats via acute ARI administration. a At 1 h following acute haloperidol (0.6 mg/kg, i.p.) administration in MAM rats, an example trace of a DA neuron demonstrates a reduction in DA neuron firing rate and bursting activity 5 min following acute ARI (1 mg/kg, i.p.) compared to before ARI. b, c Within-subject changes in average DA neuron population activity in the six tracks recorded before ARI or saline administration in HAL-treated MAM rats compared to the six tracks recorded after ARI or saline administration. d Acute ARI administration resulted in an increase in the average number of DA cells/track in HAL-treated MAM rats after ARI administration compared to before ARI administration. In contrast, there was no difference in population activity in tracks recorded post saline administration. There was no significant change in the average firing rate (e) or bursting activity (f) in DA neurons recorded before and after ARI or VEH administration. *p < 0.05
Discussion
Unlike many other clinically available APDs which exert their effects through D2 receptor antagonism, ARI is a D2 partial agonist thought to stabilize dopaminergic tone [26], although the mechanism underlying its state-dependent effect is unclear. The current study examined whether ARI reduces DA neuron activity in vivo in a normal and hyperdopaminergic system via overexcitation-induced depolarization block, as observed following D2 antagonist administration [4, 20, 35]. We demonstrate that ARI is able to state-dependently decrease the number of spontaneously active DA neurons in the VTA in the MAM rodent model of schizophrenia in a manner distinct from D2 receptor antagonists. Furthermore, after depolarization block-driven decrease in DA neuron population activity, ARI increased DA neuron population activity secondary to reversal of APD-induced depolarization block, as observed with low-dose administration of a DA agonist [4, 35]. The interpretation of these results is limited to male rats and the chosen treatment and dose regiment. Female rats were not included in the present study due to differences in DA neuron activity between female MAM and SAL rats across the estrous cycle [43] and thus would require validation of prior antipsychotic drug studies in a sex-specific manner. Examination of ARI action in female rodents across the estrous cycle is a necessary future direction, especially given reported differences in APD response between male and female patients [44] and in female patients across the menstrual cycle [45].
Aripiprazole produces a rapid and long-lasting reduction of hyperdopaminergic activity only in MAM rats
ARI, at both 3 mg/kg and 10 mg/kg, reduced DA neuron population activity in the VTA of MAM rats 2 h following oral administration without affecting SAL rats, similar to the rapid reduction of population activity in MAM rats follow D2 receptor antagonist APD administration [35]. The reduction was also present following 21-day repeated ARI treatment and persisted 7 days following withdrawal from repeated treatment, suggesting a persistent change in the DA system, as previously observed following withdrawal from repeated HAL treatment [20]. However, unlike D2 receptor antagonists [20, 35], ARI did not reduce DA neuron activity in SAL rats following repeated treatment or following 7-day withdrawal from repeated treatment. Consistent with the lack of observed change in spontaneous DA neuron activity in control rats, an in vivo microdialysis study has previously shown that acute ARI administration does not affect cortical or striatal extracellular DA levels in rats following acute and repeated oral administration [46]. Repeated oral ARI administration has also been shown to block elevated GTPase activity stimulated by the DA agonist quinpirole, but ARI alone does not stimulate GTPase activity [27]. In contrast, Li et al. [47] demonstrated a reduction in extracellular DA levels in the prefrontal cortex and nucleus accumbens following acute ARI administration at 10 mg/kg administered subcutaneously. These differences may be explained by differing administration methods, as even 21-day repeated administration of ARI 40 mg/kg p.o. has been shown to not affect striatal DA levels [46]. Our results demonstrate that ARI is able to reverse the increased DA neuron activity, as observed in MAM rats, without effect in a normal system, and that the reduction in DA neuron activity can remain stabilized following withdrawal from repeated treatment.
Aripiprazole does not reduce VTA dopamine neuron activity via depolarization block
In contrast to the reduction DA neuron population activity in MAM rats via depolarization block following acute treatment with D2 antagonists [35], the present data suggest that ARI administration does not induce depolarization block. The presence of depolarization block has been verified previously by administering low doses of the DA agonist apomorphine which leads to an inhibition-driven reactivation of neurons previously in a hyperexcitation-induced block [4, 8, 35]. In contrast, low doses of apomorphine failed to increase population activity in MAM rats following acute or following 21-day repeated ARI treatment. In addition, it did not affect population activity following 7-day withdrawal from repeated ARI treatment. However, in all cases, administration of apomorphine produced a leftward shift in the distribution of firing rate and, more prominently, bursting activity of spontaneously active DA neurons recorded post injection. In some cases, this resulted in a reduction in the number of spontaneously active DA neurons, notably observed following acute ARI administration, possibly because neurons with a previously low firing rate ceased firing. Therefore, the local feedback from the action of apomorphine presumably on D2 autoreceptors resulted in a net reduction in DA activity of ARI-treated MAM rats, rather than an increase in spontaneous activity which would be observed if the reduction in population activity was due to reversal of overexcitation-induced depolarization block. Although ARI displayed antagonist-like reduction of hyperdopaminergic activity in MAM rats, it did not appear to do so through antagonist action at postsynaptic D2 receptors that would result in depolarization block.
The question remains of how ARI reduces DA neuron population activity in MAM rats. It has been hypothesized that ARI simultaneously acts as an antagonist at D2 receptors postsynaptically and an agonist presynaptically [26, 48, 49] based on differences in receptor reserve [26]. In the presence of a receptor reserve, ARI behaves like an agonist, and in its absence, ARI predominantly displays antagonist properties [48]. The greater number of presynaptic spare receptors may underlie the autoreceptor selectivity of ARI to inhibit neuron firing [26, 48] which may have contributed to the downregulation of DA neuron population activity. In support of its agonist-like activity at autoreceptors, a reduction in both the average firing rate and bursting activity in MAM rats was observed following 7-day withdrawal from repeated treatment, although no significant reduction in firing rate or bursting was observed across the population of DA neurons recorded following acute ARI administration or following 21-day repeated ARI treatment compared to VEH-treated rats. Several other in vivo studies have demonstrated agonist-like activation of ARI at D2 autoreceptors [48, 50], including its ability to block increased DA synthesis in reserpine-treated rats [48] and a moderate decrease in the spontaneous firing of DA neurons in the VTA [51, 52]. The proposed partial agonism of ARI, with lower intrinsic activity at the receptor than a full agonist, may also account for its antagonist-like effect at postsynaptic D2 receptors in animal models of DA hyperactivity, such as blockade of apomorphine-induced hyperlocomotion and stereotypy [48]. It is difficult to conclude whether this accounts for ARI actions across a range of systems. It has been suggested from in vitro studies that ARI possesses functional selectivity with intrinsic activity dependent on the cellular environment of the receptor and the signaling pathway activated [53–56], including distinct effects on Gα and Gβγ signaling at the D2 receptor [57]. It is also possible that factors other than partial D2 agonism alone may contribute to the reduction observed following acute and repeated treatment, and/or following withdrawal from repeated treatment. For example, DA neuron population activity also remains reduced following 1 week of withdrawal from repeated haloperidol administration, but unlike the acute and repeated effects of haloperidol [4, 35], the reduction following withdrawal is not due to depolarization block and may instead be due to the modulatory influence of other brain regions [20]. Finally, ARI has a diverse receptor binding profile, including significant affinity for serotonin (5-HT)1A and 5-HT2A receptors [53, 55, 58], and the potential influence of other neurotransmitter systems on DA neuron activity cannot be excluded, though it has been argued that it does not affect 5-HT receptors at therapeutic doses [59]. Additional studies are needed to determine whether ARI downregulates DA neuron activity by acting on presynaptic D2 receptors and, furthermore, why it selectively downregulates DA neuron activity in MAM rats and not in normal rats.
Removal of haloperidol-induced depolarization block by aripiprazole
Prevalent problems associated with APD treatment and withdrawal have driven a search for treatments with a more favorable side-effect profile and improved efficacy across symptom domains. Novel target compounds for the treatment of schizophrenia have shown promise in preclinical research, but failed to show efficacy in clinical trials. However, preclinical research is typically performed on drug-naive rats, whereas clinical trials are performed on patients who have received only brief withdrawal from years of prior APD treatment. We previously found that withdrawal from repeated HAL treatment produced persistent DA supersensitivity in MAM rats, interfering with the ability of a novel target compound to reduce amphetamine-induced hyperlocomotion in MAM rats [20]. It is possible that DA supersensitivity following prior D2 antagonist treatment in patients with schizophrenia may similarly mask potential effects of novel target compounds in clinical trials, despite their promise in drug screening paradigms performed in normal, drug-naive rodents. Prior evidence that repeated ARI administration may not upregulate D2 receptors or produce DA supersensitivity [17, 27], unlike D2 receptor antagonists [13–17], indicate that ARI may circumvent this potential confound. However, in patients taking D2 antagonist APDs, the switch to ARI is reported to result in a temporary worsening of psychotic symptoms, which was suggested to be unmasking of the increase in D2 receptors from prior treatment [60, 61]. Indeed, the ability of ARI to reverse depolarization block in HAL-treated MAM rats demonstrated in the present study is consistent with the reported worsening in psychotic symptoms in patients upon transitioning to ARI from D2 antagonist drugs. Though not addressed by our experiment, this phenomenon could have interesting implications if postsynaptic D2 receptors are also upregulated, as following repeated D2 antagonist treatment, where ARI may act like an agonist both presynaptically and postsynaptically. While ARI may not have superior efficacy in schizophrenia [62, 63], it may be less likely to induce persistent deleterious effects that can arise from a persistent elevation in DA sensitivity that occurs with D2 antagonists [16, 64].
Overall, this study demonstrates that ARI rapidly normalizes the hyperdopaminergic state observed in MAM rats without effect on DA neuron population activity in a normal system. In contrast to the action of D2 receptor antagonists, the reduction is unlikely due to depolarization block. Although it is unknown how ARI reduces DA neuron activity in MAM rats, it may act as an agonist on presynaptic receptors to downregulate spontaneous activity.
Acknowledgements
We thank Niki MacMurdo and Christy Smolak for their technical assistance and Sarah Miller for her assistance in drug administration and electrophysiological recordings. This work was supported by the National Institute of Health (grant numbers MH57440 and MH104320).
Competing interests
SFS and KMG declare no competing interests. AAG received funds from Lundbeck, Pfizer, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, Alkermes, and Newron.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 2.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–9. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 3.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–71. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 4.Grace A, Bunney B. Induction of depolarization block in midbrain dopamine neurons by repeated administration of haloperidol: analysis using in vivo intracellular recording. J Pharmacol Exp Ther. 1986;238:1092–100. [PubMed] [Google Scholar]
- 5.Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20:31–7. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- 6.Pucak ML, Grace AA. Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J Pharmacol Exp Ther. 1994;271:1181–92. [PubMed] [Google Scholar]
- 7.Pucak ML, Grace AA. Effects of haloperidol on the activity and membrane physiology of substantia nigra dopamine neurons recorded in vitro. Brain Res. 1996;713:44–52. doi: 10.1016/0006-8993(95)01460-8. [DOI] [PubMed] [Google Scholar]
- 8.Bunney B, Grace A. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 1978;23:1715–27. doi: 10.1016/0024-3205(78)90471-X. [DOI] [PubMed] [Google Scholar]
- 9.Braszko J, Bannon MJ, Bunney BS, Roth RH. Intrastriatal kainic acid: acute effects on electrophysiological and biochemical measures of nigrostriatal dopaminergic activity. J Pharmacol Exp Ther. 1981;216:289–93. [PubMed] [Google Scholar]
- 10.Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3:1607–19. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 12.Lane RF, Blaha CD. Chronic haloperidol decreases dopamine release in striatum and nucleus accumbens in vivo: depolarization block as a possible mechanism of action. Brain Res Bull. 1987;18:135–8. doi: 10.1016/0361-9230(87)90042-6. [DOI] [PubMed] [Google Scholar]
- 13.Lidow MS, Goldman-Rakic PS. A common action of clozapine, haloperidol, and remoxipride on D1-and D2-dopaminergic receptors in the primate cerebral cortex. Proc Natl Acad Sci USA. 1994;91:4353–6. doi: 10.1073/pnas.91.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Dell SJ, Lahoste GJ, Widmark CB, Shapiro RM, Potkin SG, Marshall JF. Chronic treatment with clozapine or haloperidol differentially regulates dopamine and serotonin receptors in rat brain. Synapse. 1990;6:146–53. doi: 10.1002/syn.890060205. [DOI] [PubMed] [Google Scholar]
- 15.See R, Toga A, Ellison G. Autoradiographic analysis of regional alterations in brain receptors following chronic administration and withdrawal of typical and atypical neuroleptics in rats. J Neural Transm. 1990;82:93–109. doi: 10.1007/BF01245166. [DOI] [PubMed] [Google Scholar]
- 16.Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi C, Remington GJ, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl) 2000;152:174–80. doi: 10.1007/s002130000532. [DOI] [PubMed] [Google Scholar]
- 17.Tadokoro S, Okamura N, Sekine Y, Kanahara N, Hashimoto K, Iyo M. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2011;38:1012–20. doi: 10.1093/schbul/sbr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chouinard G, Jones BD. Neuroleptic-induced supersensitivity psychosis: clinical and pharmacologic characteristics. Am J Psychiatry. 1980;137:16–21. doi: 10.1176/ajp.137.8.992-a. [DOI] [PubMed] [Google Scholar]
- 19.Chouinard G, Chouinard VA. Atypical antipsychotics: CATIE study, drug-induced movement disorder and resulting iatrogenic psychiatric-like symptoms, supersensitivity rebound psychosis and withdrawal discontinuation syndromes. Psychother Psychosom. 2008;77:69–77. doi: 10.1159/000112883. [DOI] [PubMed] [Google Scholar]
- 20.Gill KM, Cook JM, Poe MM, Grace AA. Prior antipsychotic drug treatment prevents response to novel antipsychotic agent in the methylazoxymethanol acetate model of schizophrenia. Schizophr Bull. 2014;40:341–50. doi: 10.1093/schbul/sbt236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montanaro N, Dall’Olio R, Gandolfi O, Vaccheri A. Differential enhancement of behavioral sensitivity to apomorphine following chronic treatment of rats with (−)-sulpiride and haloperidol. Eur J Pharmacol. 1982;81:1–9. doi: 10.1016/0014-2999(82)90595-7. [DOI] [PubMed] [Google Scholar]
- 22.Samaha AN, Seeman P, Stewart J, Rajabi H, Kapur S. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27:2979–86. doi: 10.1523/JNEUROSCI.5416-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samaha AN, Reckless GE, Seeman P, Diwan M, Nobrega JN, Kapur S. Less is more: antipsychotic drug effects are greater with transient rather than continuous delivery. Biol Psychiatry. 2008;64:145–52. doi: 10.1016/j.biopsych.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Wilmot CA, Szczepanik AM. Effects of acute and chronic treatments with clozapine and haloperidol on serotonin (5-HT 2) and dopamine (D 2) receptors in the rat brain. Brain Res. 1989;487:288–98. doi: 10.1016/0006-8993(89)90833-0. [DOI] [PubMed] [Google Scholar]
- 25.Grace A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-U. [DOI] [PubMed] [Google Scholar]
- 26.Burris K, Molski T, Xu C, Ryan E, Tottori K, Kikuchi T, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–9. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 27.Inoue A, Miki S, Seto M, Kikuchi T, Morita S, Ueda H, et al. Aripiprazole, a novel antipsychotic drug, inhibits quinpriole-evoked GTPase activity but does not up-regulate dopamine D 2 receptor following repeated treatment in the rat striatum. Eur J Pharmacol. 1997;321:105–11. doi: 10.1016/S0014-2999(96)00920-X. [DOI] [PubMed] [Google Scholar]
- 28.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–30. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–54. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modinos G, Allen P, Grace AA, McGuire P. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38:129–38. doi: 10.1016/j.tins.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–64. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kegeles L, Shungu D, Anjilvel S, Chan S, Ellis S, Xanthopoulos E, et al. Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry Res. 2000;98:163–75. doi: 10.1016/S0925-4927(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 33.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–46. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valenti O, Cifelli P, Gill KM, Grace AA. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J Neurosci. 2011;31:12330–8. doi: 10.1523/JNEUROSCI.2808-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson SA, Boctor SY. Use of food wafers for multiple daily oral treatments in young rats. J Am Assoc Lab Anim Sci. 2009;48:292–5. [PMC free article] [PubMed] [Google Scholar]
- 37.Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38:1881. doi: 10.1038/npp.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimokawa Y, Akiyama H, Kashiyama E, Koga T, Miyamoto G. High performance liquid chromatographic methods for the determination of aripiprazole with ultraviolet detection in rat plasma and brain: application to the pharmacokinetic study. J Chromatogr B. 2005;821:8–14. doi: 10.1016/j.jchromb.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Grace A, Bunney B. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–15. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 40.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–90. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–30. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Beijing: Qingchuan Zhuge translate People’s Medical Publishing House; 2007. p. 32. [Google Scholar]
- 43.Perez SM, Chen L, Lodge DJ. Alterations in dopamine system function across the estrous cycle of the MAM rodent model of schizophrenia. Psychoneuroendocrinology. 2014;47:88–97. doi: 10.1016/j.psyneuen.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein JM, Cohen LS, Horton NJ, Lee H, Andersen S, Tohen M, et al. Sex differences in clinical response to olanzapine compared with haloperidol. Psychiatry Res. 2002;110:27–37. doi: 10.1016/S0165-1781(02)00028-8. [DOI] [PubMed] [Google Scholar]
- 45.Bergemann N, Parzer P, Runnebaum B, Resch F, Mundt C. Estrogen, menstrual cycle phases, and psychopathology in women suffering from schizophrenia. Psychol Med. 2007;37:1427–36. doi: 10.1017/S0033291707000578. [DOI] [PubMed] [Google Scholar]
- 46.Jordan S, Koprivica V, Dunn R, Tottori K, Kikuchi T, Altar CA. In vivo effects of aripiprazole on cortical and striatal dopaminergic and serotonergic function. Eur J Pharmacol. 2004;483:45–53. doi: 10.1016/j.ejphar.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Ichikawa J, Dai J, Meltzer HY. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur J Pharmacol. 2004;493:75–83. doi: 10.1016/j.ejphar.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 48.Kikuchi T, Tottori K, Uwahodo Y, Hirose T, Miwa T, Oshiro Y, et al. 7-(4-[4-(2, 3-Dichlorophenyl)-1-piperazinyl] butyloxy)-3, 4-dihydro-2 (1H)-quinolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther. 1995;274:329–36. [PubMed] [Google Scholar]
- 49.Oshiro Y, Sato S, Kurahashi N, Tanaka T, Kikuchi T, Tottori K, et al. Novel antipsychotic agents with dopamine autoreceptor agonist properties: synthesis and pharmacology of 7-[4-(4-phenyl-1-piperazinyl) butoxy]-3, 4-dihydro-2 (1 H)-quinolinone derivatives. J Med Chem. 1998;41:658–67. doi: 10.1021/jm940608g. [DOI] [PubMed] [Google Scholar]
- 50.Semba J, Watanabe A, Kito S, Toru M. Behavioural and neurochemical effects of OPC-14597, a novel antipsychotic drug, on dopaminergic mechanisms in rat brain. Neuropharmacology. 1995;34:785–91. doi: 10.1016/0028-3908(95)00059-F. [DOI] [PubMed] [Google Scholar]
- 51.Bortolozzi A, Diaz-Mataix L, Toth M, Celada P, Artigas F. In vivo actions of aripiprazole on serotonergic and dopaminergic systems in rodent brain. Psychopharmacology (Berl) 2007;191:745–58. doi: 10.1007/s00213-007-0698-y. [DOI] [PubMed] [Google Scholar]
- 52.Momiyama T, Amano T, Todo N, Sasa M. Inhibition by a putative antipsychotic quinolinone derivative (OPC-14597) of dopaminergic neurons in the ventral tegmental area. Eur J Pharmacol. 1996;310:1–8. doi: 10.1016/0014-2999(96)00350-0. [DOI] [PubMed] [Google Scholar]
- 53.Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, et al. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–27. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 54.Mailman RB, Gay EA. Novel mechanisms of drug action: functional selectivity at D2 dopamine receptors. Med Chem Res. 2004;13:115–26. doi: 10.1007/s00044-004-0017-7. [DOI] [Google Scholar]
- 55.Shapiro DA, Renock S, Arrington E, Chiodo LA, Li-Xin L, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 56.Urban JD, Vargas GA, Von Zastrow M, Mailman RB. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 2007;32:67–77. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- 57.Brust TF, Hayes MP, Roman DL, Watts VJ. New functional activity of aripiprazole revealed: Robust antagonism of D2 dopamine receptor-stimulated Gβγ signaling. Biochem Pharmacol. 2015;1:85–91. doi: 10.1016/j.bcp.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol. 2002;441:137–40. doi: 10.1016/S0014-2999(02)01532-7. [DOI] [PubMed] [Google Scholar]
- 59.Wood M, Reavill C. Aripiprazole acts as a selective dopamine D2 receptor partial agonist. Expert Opin Investig Drugs. 2007;16:771–5. doi: 10.1517/13543784.16.6.771. [DOI] [PubMed] [Google Scholar]
- 60.Tadokoro S, Nonomura N, Kanahara N, Hashimoto K, Iyo M. Reduction of severity of recurrent psychotic episode by sustained treatment with aripiprazole in a schizophrenic patient with dopamine supersensitivity: a case report. Clin Psychopharmacol Neurosci. 2017;15:79. doi: 10.9758/cpn.2017.15.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takase M, Kanahara N, Oda Y, Kimura H, Watanabe H, Iyo M. Dopamine supersensitivity psychosis and dopamine partial agonist: a retrospective survey of failure of switching to aripiprazole in schizophrenia. J Psychopharmacol. 2015;29:383–9. doi: 10.1177/0269881115570083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 63.Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166:152–63. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- 64.Chouinard G, Samaha AN, Chouinard VA, Peretti CS, Kanahara N, Takase M, et al. Antipsychotic-induced dopamine supersensitivity psychosis: pharmacology, criteria, and therapy. Psychother Psychosom. 2017;86:189–19. doi: 10.1159/000477313. [DOI] [PubMed] [Google Scholar]