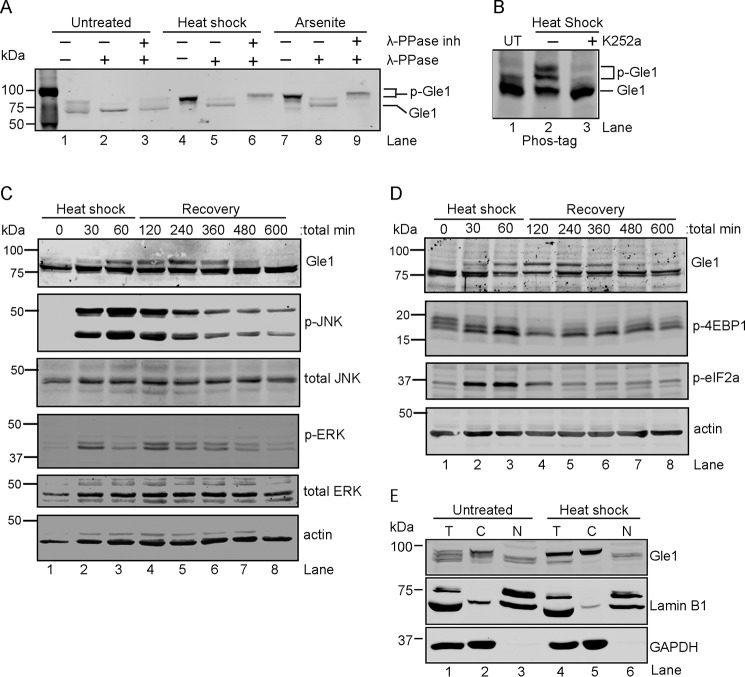
Figure 1.
Cytoplasmic Gle1 is dynamically phosphorylated in response to stress. A and B, Gle1 is phosphorylated during stress. A, HeLa cells were either left untreated, exposed to heat shock at 45 °C, or treated with 0.5 mm sodium arsenite for 60 min. Cell lysates were prepared and incubated with either phosphatase buffer alone, λ-PPase, or λ-PPase and phosphatase inhibitors together for 30 min. Samples were resolved by SDS-PAGE and immunoblotted with anti-Gle1 antibodies. B, HeLa cells were either left untreated (UT) or preincubated with the broad-spectrum kinase inhibitor K252a (1 μm) for 60 min at 37 °C followed by heat shock at 45 °C for 60 min. Cell lysates were resolved on a Phos-tag SDS-PAGE gel and immunoblotted with anti-Gle1 antibodies. C and D, Gle1 phosphorylation follows MAPK activation (C) and phosphorylation (p) of translation factors (D). HeLa cells subjected to heat shock at 45 °C for 60 min followed by a recovery phase at 37 °C were harvested at the indicated time points. Lysates were analyzed by immunoblotting using the indicated antibodies. E, phosphorylated Gle1 localizes to the cytoplasm. Following heat shock at 45 °C for 60 min, nuclear (N) and cytosolic (C) HeLa cell fractions were prepared, resolved by SDS-PAGE, and immunoblotted with anti-Gle1, anti-lamin B1, and anti-GAPDH antibodies. T, total cell lysate.