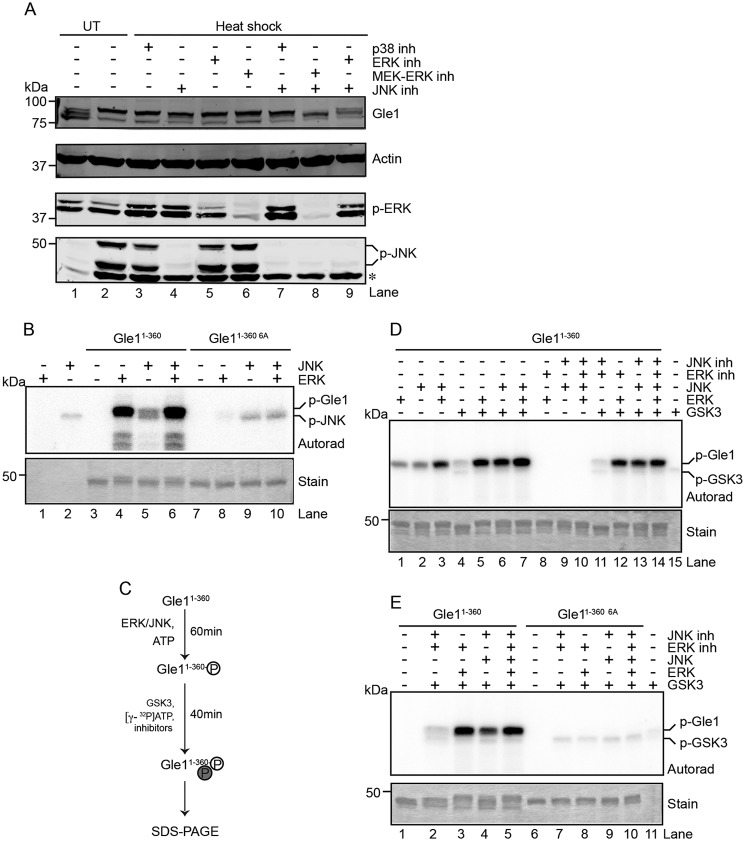
Figure 3.
Phosphorylation events by the MAPKs ERK and JNK prime GSK3 to phosphorylate Gle1. A and B, ERK and JNK phosphorylate Gle1 in the N-terminal Ser88–Thr102 cluster. A, HeLa cells were untreated (UT) or heat-shocked at 45 °C for 60 min in the presence of the indicated kinase inhibitors (inh) (p38 (SB203580), ERK (FR180204), MEK-ERK (U0126), and/or JNK (JNK-IN-8)), lysed, and immunoblotted with the indicated antibodies. The asterisk indicates a nonspecific band detected by anti-phospho (p)-JNK antibody. B, bacterially expressed and purified recombinant Gle11–360 or gle11–360 6A proteins were incubated with recombinant active ERK and/or JNK in the presence of radioactive [γ-32P]ATP. Reactions were terminated by addition of Laemmli sample denaturation buffer and resolved by SDS-PAGE, and radioisotope incorporation was measured by autoradiography (Autorad). Coomassie stain demonstrates equivalent protein loading. C–E, phosphorylation of Gle1 by ERK and JNK primes GSK3 to phosphorylate Gle1. C, schematic diagram describes the sequential in vitro kinase assay. D and E, GSK3 phosphorylation occurs in the N terminus of Gle1 within the Ser88–Thr102 cluster of phosphorylation sites. D, purified Gle11–360 was preincubated with ERK and JNK followed by the addition of active recombinant GSK3 in the presence of [γ-32P]ATP. ERK and JNK activity was inhibited during GSK3 incubation as indicated, and samples were analyzed for phosphorylation as in B. E, sequential in vitro kinase reactions were performed as in D for purified Gle11–360 or gle11–360 6A.