Figure 4.
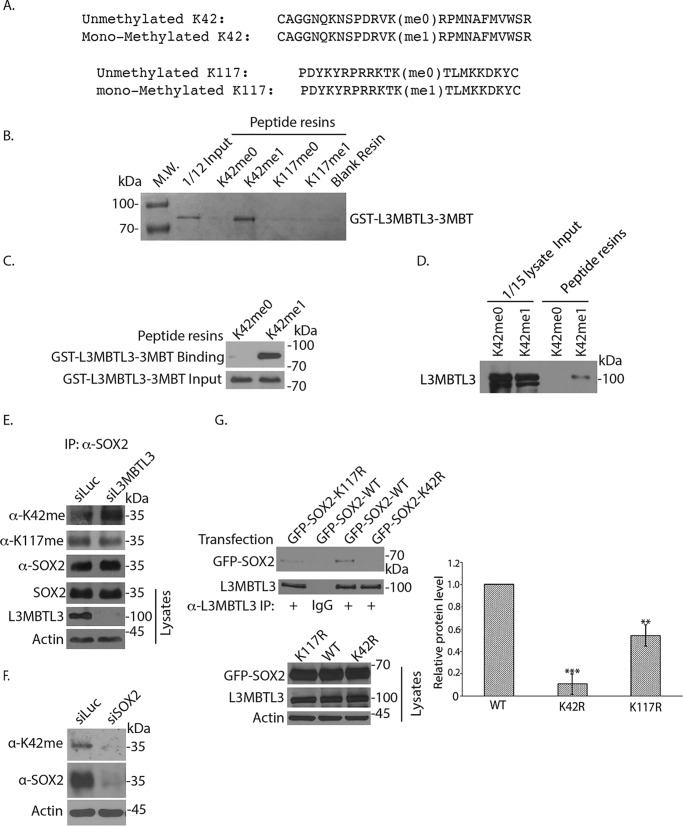
L3MBTL3 preferentially binds to the methylated Lys-42 (K42) in SOX2. A, two pairs of synthetic SOX2 peptides were immobilized onto Sulfolink-Sepharose through the cysteine residues at the ends of the peptides. One pair of SOX2 peptides contains the monomethylated Lys-42 (Kme1) or the cognate peptide with unmethylated Lys-42 (Kme0); another pair contains the monomethylated Lys-117 (Kme1) or cognate unmethylated Lys-117 (Kme0) as indicated. B, L3MBTL3 directly interacts with the monomethylated Lys-42 peptide. The K42me0, K42me1, K117me0, and K117me1 peptide resins were preincubated with 5 μg of GST for 2 h to block the background. The resins were then incubated with 1.5 μg of GST-L3MBTL3–3MBT recombinant protein for 4 h at 4 °C and then washed. The GST-L3MBTL3–3MBT protein associated with various resins was separated in protein gel and visualized by Coomassie Blue staining. C, the K42me0 and K42me1 peptide resins were incubated with GST-L3MBTL3–3MBT recombinant protein as in B. The proteins associated with the resins were blotted with anti-L3MBTL3 antibodies. D, endogenous L3MBTL3 preferentially binds to the monomethylated Lys-42 peptide. The monomethylated (Kme1) or cognate unmethylated Lys-42 (Kme0) peptide resins were incubated with 250 μg of PA-1 cell lysates for 4 h. L3MBTL3 binding to the peptide resins was analyzed by the anti-L3MBTL3 Western blotting. E, loss of L3MBTL3 caused the accumulation of methylated Lys-42 in SOX2. PA-1 cells were transfected with 50 nm luciferase (control) and L3MBTL3 siRNAs for 48 h. SOX2 were immunoprecipitated (IP) by SOX2 antibodies and Western blotted with affinity-purified anti-methylated Lys-42, anti-methylated Lys-117, and SOX2 antibodies. F, PA-1 cells were transfected with 50 nm luciferase (control) and SOX2 siRNAs for 48 h. SOX2 and Lys-42–methylated SOX2 protein were Western blotted with affinity-purified anti-methylated Lys-42 and SOX2 antibodies in cell lysates. G, GFP-tagged SOX2 (WT), K42R, and K117R mutant SOX2 expression constructs were transfected into 293 cells. The L3MBTL3 complexes were immunoprecipitated by anti-L3MBTL3 antibodies and Western blotted by anti-SOX2 antibodies. The proteins bands were quantified and normalized to the WT SOX2 band. The quantifications are represented by a bar graph with mean and S.D. (error bars) from replicated experiments. The p value of K117R or K42R bands to the WT SOX2 band was calculated by independent Student's t test (**, p < 0.01; ***, p < 0.001). Experiments were repeated with the same conclusion (see Fig. S1C).