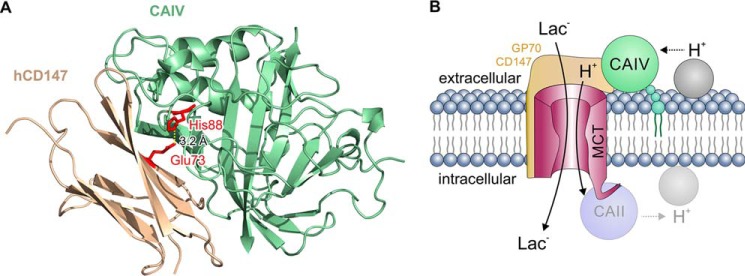
Figure 9.
A, structural model of the direct interaction between CAIV and CD147. CAIV (green cartoon) binds to the Ig1 domain of hCD147 (ochre cartoon) by formation of a hydrogen bond (dotted line) between CAIV–His-88 and CD147–Glu-73 (red sticks) with a distance of 3.2 Å. B, hypothetical model of the functional interaction between MCT, CD147/GP70, and CAIV. CAIV (green circle), which is tethered to the extracellular site of the plasma membrane via a GPI anchor (small green circles), binds MCTs via the Ig1 domain of their chaperones CD147 and GP70 (light ochre structure). This binding brings CAIV close enough to the transporter pore to shuttle protons between transporter and surrounding protonable residues (gray circle). On the intracellular site, CAII (light blue circle), which binds to the C-terminal tail of MCT1 and MCT4 (57, 62, 63), facilitates the exchange of protons between transporter and intracellular protonable residues (light gray circle) in a similar fashion as CAIV. By this noncatalytic mechanism, intracellular and extracellular carbonic anhydrases could facilitate proton-coupled lactate flux across the cell membrane.